Abstract
AIMS—To determine if interleukin 2 (IL-2) alters epithelial transport and barrier function in cultured human small intestinal enterocytes. METHODS—Confluent monolayers of small intestinal cells derived from duodenal biopsies were treated with IL-2 0.2-50 U/ml for 24 hours prior to study. Transport measurements were performed under short circuited conditions in Ussing chambers, with and without the secretagogues forskolin and 3-isobutyl-1-methyl xanthine (IBMX). Serosal to mucosal flux of 3[H] mannitol (permeability) and 3[H] thymidine uptake (proliferation) were measured. IL-2 receptor and cystic fibrosis transmembrane conductance regulator (CFTR) mRNA were identified using reverse transcription-polymerase chain reaction (RT-PCR). RESULTS—IL-2 did not alter baseline electrical parameters but caused a significant increase in cAMP dependent chloride secretion. The effect was mediated by the IL-2 receptor and paralleled a rapid increase in tyrosine phosphorylation, janus kinase 1, and signal transducers and activators of transcription (STATs) 1, 3, and 5. IL-2 significantly increased proliferation but at a lower dose than observed for enhanced secretion but did not alter permeability. IL-2 receptor β and γc chains and CFTR mRNA were identified by RT-PCR. CONCLUSIONS—IL-2 treatment enhances cAMP stimulated chloride secretion and cellular proliferation in a human small intestinal cell line expressing a functional IL-2 receptor. Keywords: interleukin 2; ion secretion; cell proliferation; enterocytes; small intestine
Full Text
The Full Text of this article is available as a PDF (230.4 KB).
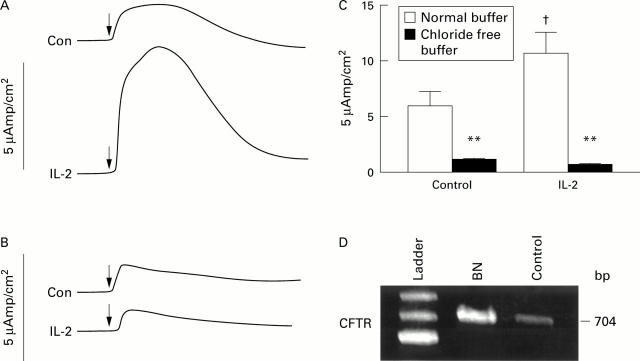
Figure 1 .
Effect of interleukin 2 (IL-2) on cAMP dependent chloride secretion in BN cells. (A) Representative traces of control (Con) and IL-2 treated monolayers. (B) Representative traces of control and IL-2 treated monolayers in chloride free buffer. (C) Secretagogue stimulated short circuit current (ΔIsc) in normal buffer and paired monolayers in chloride free buffer (n=7-9 pairs, data are mean (SEM)). (D) Constitutive expression of cystic fibrosis transmembrane conductance regulator (CFTR) mRNA in BN cells and controls (human small intestinal biopsy). Cells depicted in (A-C) were treated with IL-2 5 U/ml for 24 hours prior to the study; secretion, stimulated with 3-isobutyl-1-methyl xanthine (IBMX) 3×10−4 M and forskolin 10−6 M (arrows). bp, base pairs. **p<0.01 compared with control and †p<0.02 compared with respective control period by paired t test.
Figure 2 .
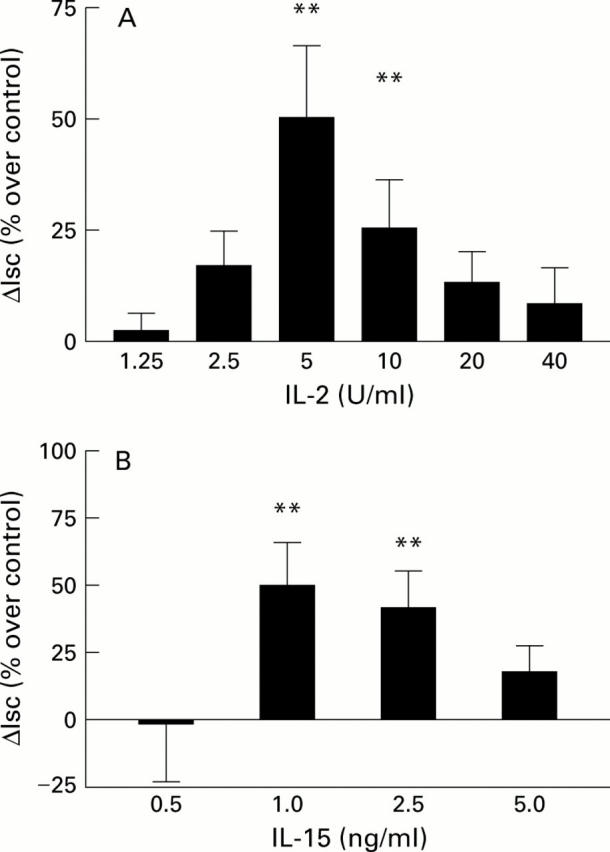
Dose response of (A) interleukin 2 (IL-2) and (B) interleukin 15 (IL-15) secretagogue stimulated short circuit current (Isc). Cells were incubated for 24 hours with either IL-2 or IL-15. Anion secretion was stimulated with forskolin 10−5 M and 3-isobutyl-1-methyl xanthine (IBMX) 3×10−4 M. Data are mean (SEM). Change in Isc (ΔIsc) is depicted as percentage of paired control monolayer, n=14-31 pairs. **p<0.01 compared with control by paired t test.
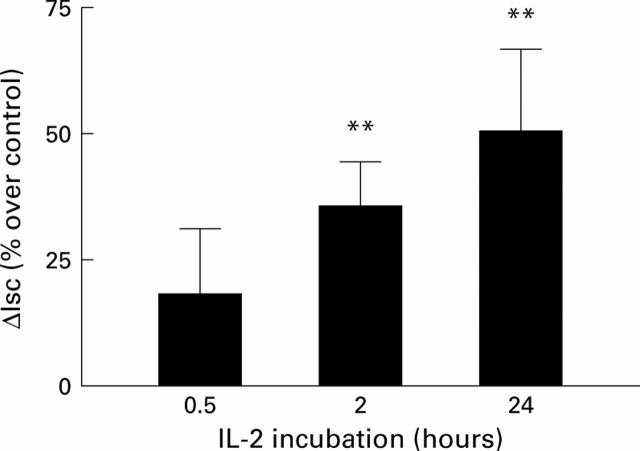
Figure 3 .
Time course of interleukin 2 (IL-2) effect on secretagogue stimulated short circuit current (Isc). Cells were treated with IL-2 5 U/ml. Anion secretion was stimulated with forskolin 10−5 M and 3-isobutyl-1-methyl xanthine (IBMX) 3×10−4 M. **p<0.01 compared with paired control monolayers. n=7, 14, and 23 pairs at 0.5, 2, and 24 hours, respectively.
Figure 4 .
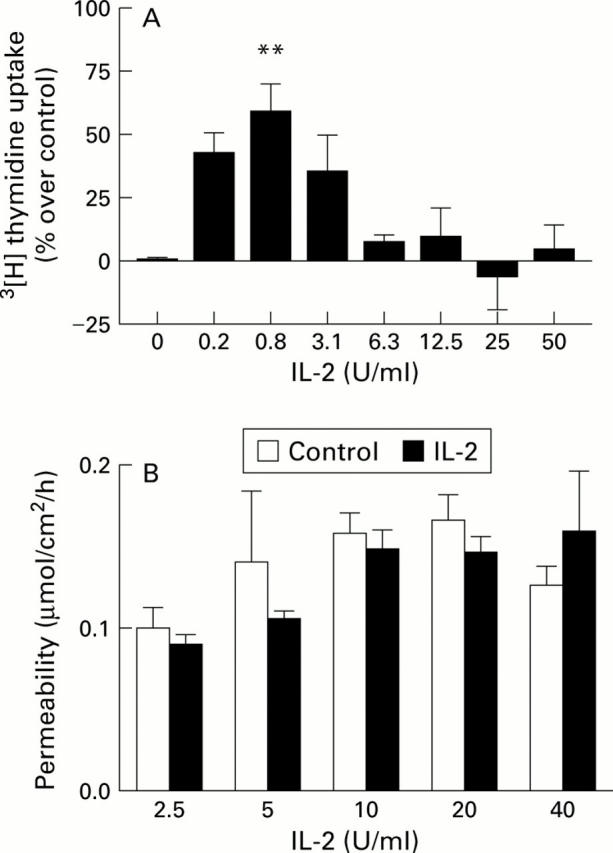
Dose-response of interleukin 2 (IL-2) effect on (A) cellular proliferation and (B) mannitol permeability. In (A), n=3 dose-response experiments. **p<0.01 compared with untreated monolayers. In (B), mannitol fluxes are serosal to mucosal in n=13-23 pairs.
Figure 5 .
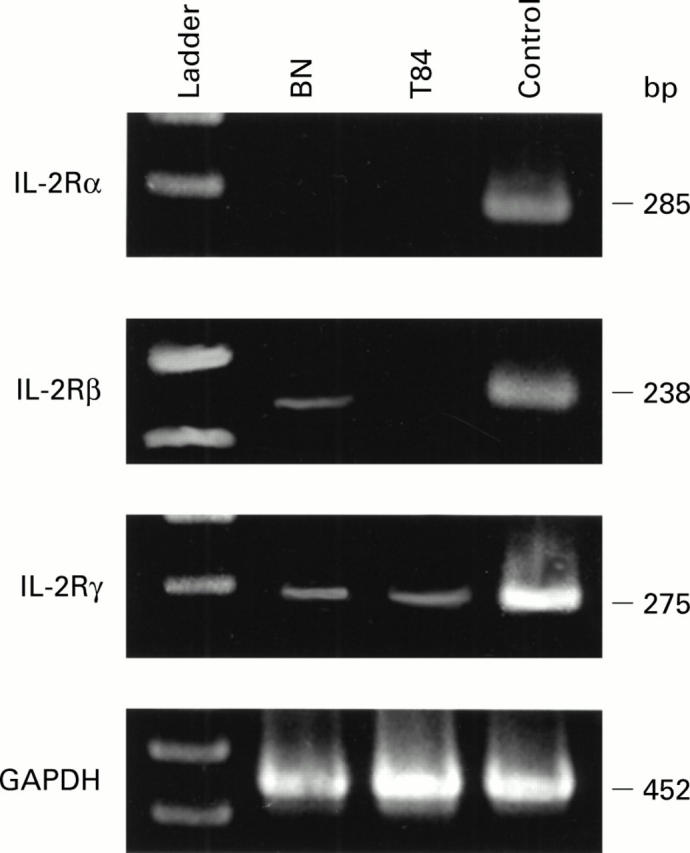
Constitutive expression of interleukin 2 receptor (IL-2R) α, β, and γ chain mRNA genes. Control transcripts were derived from peripheral blood lymphocytes and exhibited all three chains of the receptor. In contrast, BN (small intestinal cells) expressed β and γ chains, and T84 cells γ chain only. bp, base pairs; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 6 .
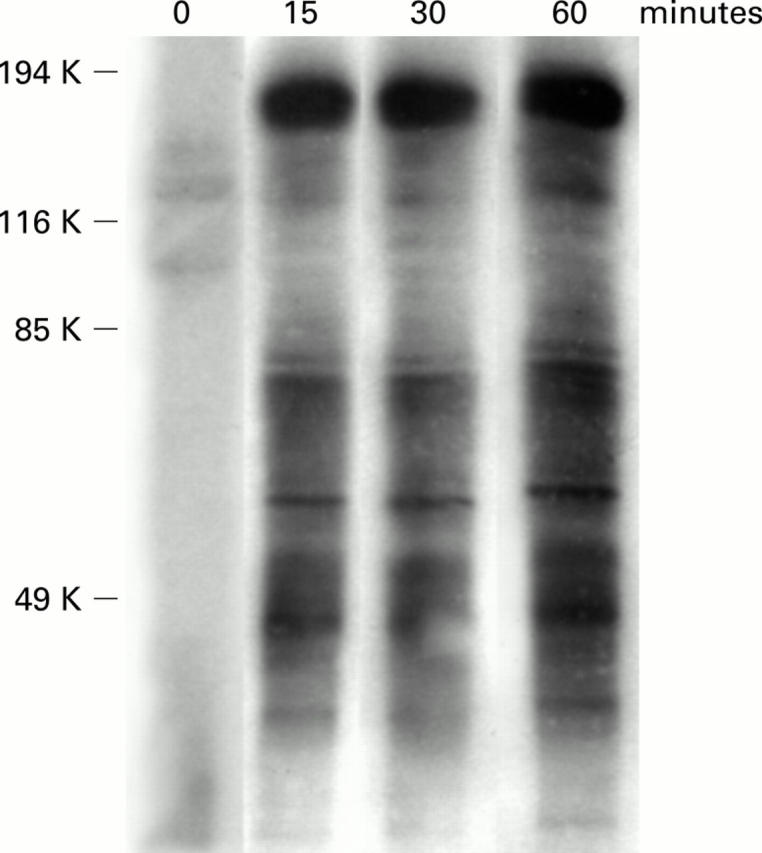
Time dependent tyrosine phosphorylation of proteins in BN cells treated with interleukin 2 (IL-2) 5 U/ml. Numbers on the left indicate molecular weight. Multiple bands of tyrosine phosphorylated proteins were detected by 15 minutes.
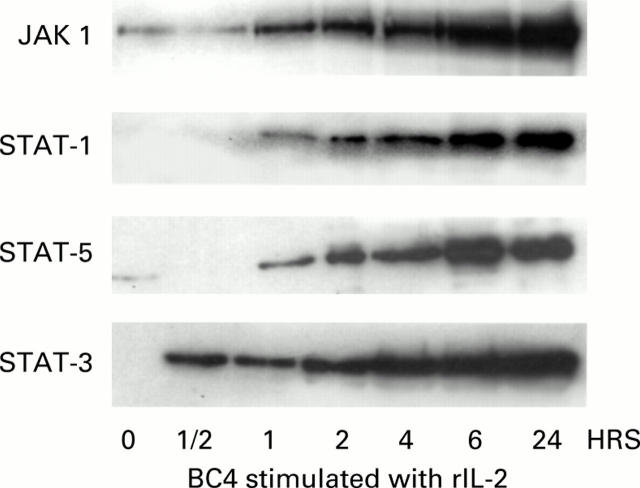
Figure 7 .
Time dependent increase in expression of janus kinase 1 (JAK1) and signal transducers and activators of transcription (STAT)-1, -3, and -5 in BN cells treated with interleukin 2 (IL-2) 5 U/ml. Cells were treated with IL-2 and lysed at various time points from 0.5 to 24 hours of treatment and proteins detected as described under methods. IL-2 treatment increased expression of all proteins from 30 to 60 minutes post incubation.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. B., Planchon S. M., Roche J. K. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993 Mar 15;150(6):2356–2363. [PubMed] [Google Scholar]
- Arcari P., Martinelli R., Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Res. 1984 Dec 11;12(23):9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaha S., Bell C. J., Wallace J. L., MacNaughton W. K. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am J Physiol. 1999 Mar;276(3 Pt 1):G703–G710. doi: 10.1152/ajpgi.1999.276.3.G703. [DOI] [PubMed] [Google Scholar]
- Besançon F., Przewlocki G., Baró I., Hongre A. S., Escande D., Edelman A. Interferon-gamma downregulates CFTR gene expression in epithelial cells. Am J Physiol. 1994 Nov;267(5 Pt 1):C1398–C1404. doi: 10.1152/ajpcell.1994.267.5.C1398. [DOI] [PubMed] [Google Scholar]
- Boismenu R., Havran W. L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994 Nov 18;266(5188):1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., McRoberts J. A., Mandel K. G., Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest. 1985 Nov;76(5):1837–1842. doi: 10.1172/JCI112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro G. A., Harari Y., Russell D. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol. 1987 Oct;253(4 Pt 1):G540–G548. doi: 10.1152/ajpgi.1987.253.4.G540. [DOI] [PubMed] [Google Scholar]
- Catto-Smith A. G., Patrick M. K., Hardin J. A., Gall D. G. Intestinal anaphylaxis in the rat: mediators responsible for the ion transport abnormalities. Agents Actions. 1989 Nov;28(3-4):185–191. doi: 10.1007/BF01967399. [DOI] [PubMed] [Google Scholar]
- Chang E. B., Musch M. W., Mayer L. Interleukins 1 and 3 stimulate anion secretion in chicken intestine. Gastroenterology. 1990 Jun;98(6):1518–1524. doi: 10.1016/0016-5085(90)91084-j. [DOI] [PubMed] [Google Scholar]
- Ciacci C., Mahida Y. R., Dignass A., Koizumi M., Podolsky D. K. Functional interleukin-2 receptors on intestinal epithelial cells. J Clin Invest. 1993 Jul;92(1):527–532. doi: 10.1172/JCI116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciacci C., Mahida Y. R., Dignass A., Koizumi M., Podolsky D. K. Functional interleukin-2 receptors on intestinal epithelial cells. J Clin Invest. 1993 Jul;92(1):527–532. doi: 10.1172/JCI116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E., Li Z., Bell C., Stiel D., Buret A., Wallace J., Brzuszczak I., O'Loughlin E. Modulation of host response to Escherichia coli o157:H7 infection by anti-CD18 antibody in rabbits. Gastroenterology. 1994 Jun;106(6):1554–1561. doi: 10.1016/0016-5085(94)90410-3. [DOI] [PubMed] [Google Scholar]
- Ferreira R. C., Forsyth L. E., Richman P. I., Wells C., Spencer J., MacDonald T. T. Changes in the rate of crypt epithelial cell proliferation and mucosal morphology induced by a T-cell-mediated response in human small intestine. Gastroenterology. 1990 May;98(5 Pt 1):1255–1263. doi: 10.1016/0016-5085(90)90342-x. [DOI] [PubMed] [Google Scholar]
- Hinterleitner T. A., Saada J. I., Berschneider H. M., Powell D. W., Valentich J. D. IL-1 stimulates intestinal myofibroblast COX gene expression and augments activation of Cl- secretion in T84 cells. Am J Physiol. 1996 Oct;271(4 Pt 1):C1262–C1268. doi: 10.1152/ajpcell.1996.271.4.C1262. [DOI] [PubMed] [Google Scholar]
- Ishii N., Takeshita T., Kimura Y., Tada K., Kondo M., Nakamura M., Sugamura K. Expression of the IL-2 receptor gamma chain on various populations in human peripheral blood. Int Immunol. 1994 Aug;6(8):1273–1277. doi: 10.1093/intimm/6.8.1273. [DOI] [PubMed] [Google Scholar]
- Kelly C. P., Becker S., Linevsky J. K., Joshi M. A., O'Keane J. C., Dickey B. F., LaMont J. T., Pothoulakis C. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Invest. 1994 Mar;93(3):1257–1265. doi: 10.1172/JCI117080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton W. K., Lowe S. S., Cushing K. Role of nitric oxide in inflammation-induced suppression of secretion in a mouse model of acute colitis. Am J Physiol. 1998 Dec;275(6 Pt 1):G1353–G1360. doi: 10.1152/ajpgi.1998.275.6.G1353. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Parkos C., Colgan S., MacLeod R. J., Nash S., Matthews J., Delp C., Lencer W. Cl- secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J Clin Invest. 1992 Jun;89(6):1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Patapoff T. W., Gillece-Castro B., Colgan S. P., Parkos C. A., Delp C., Mrsny R. J. 5'-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993 May;91(5):2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen K. L., Lewis S. A., Tavernini M. M., Hibbard J., Fedorak R. N. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997 Jul;113(1):151–159. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- Madsen K. L., Tavernini M. M., Mosmann T. R., Fedorak R. N. Interleukin 10 modulates ion transport in rat small intestine. Gastroenterology. 1996 Oct;111(4):936–944. doi: 10.1016/s0016-5085(96)70061-6. [DOI] [PubMed] [Google Scholar]
- Martin D. K., Bootcov M. R., Campbell T. J., French P. W., Breit S. N. Human macrophages contain a stretch-sensitive potassium channel that is activated by adherence and cytokines. J Membr Biol. 1995 Oct;147(3):305–315. doi: 10.1007/BF00234528. [DOI] [PubMed] [Google Scholar]
- McKay D. M., Croitoru K., Perdue M. H. T cell-monocyte interactions regulate epithelial physiology in a coculture model of inflammation. Am J Physiol. 1996 Feb;270(2 Pt 1):C418–C428. doi: 10.1152/ajpcell.1996.270.2.C418. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C., Blackmon D. L., Hamosh A., Oliva M. M., Hawkins A. L., Curristin S. M., Griffin C. A., Yang V. W., Guggino W. B., Cutting G. R. Regulation of cystic fibrosis transmembrane conductance regulator (CFTR) gene transcription and alternative RNA splicing in a model of developing intestinal epithelium. J Biol Chem. 1992 Sep 25;267(27):19299–19305. [PubMed] [Google Scholar]
- Nakamura Y., Russell S. M., Mess S. A., Friedmann M., Erdos M., Francois C., Jacques Y., Adelstein S., Leonard W. J. Heterodimerization of the IL-2 receptor beta- and gamma-chain cytoplasmic domains is required for signalling. Nature. 1994 May 26;369(6478):330–333. doi: 10.1038/369330a0. [DOI] [PubMed] [Google Scholar]
- Nakarai T., Robertson M. J., Streuli M., Wu Z., Ciardelli T. L., Smith K. A., Ritz J. Interleukin 2 receptor gamma chain expression on resting and activated lymphoid cells. J Exp Med. 1994 Jul 1;180(1):241–251. doi: 10.1084/jem.180.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Loughlin E. V., Hunt D. M., Kreutzmann D. Postnatal development of colonic electrolyte transport in rabbits. Am J Physiol. 1990 Mar;258(3 Pt 1):G447–G453. doi: 10.1152/ajpgi.1990.258.3.G447. [DOI] [PubMed] [Google Scholar]
- O'Loughlin E., Winter M., Shun A., Hardin J. A., Gall D. G. Structural and functional adaptation following jejunal resection in rabbits: effect of epidermal growth factor. Gastroenterology. 1994 Jul;107(1):87–93. doi: 10.1016/0016-5085(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Takeshita T., Nagata K., Mori S., Sugamura K. Differential expression of the IL-2 receptor subunits, p55 and p75 on various populations of primary peripheral blood mononuclear cells. J Immunol. 1989 Dec 1;143(11):3548–3555. [PubMed] [Google Scholar]
- Pang G., Buret A., O'Loughlin E., Smith A., Batey R., Clancy R. Immunologic, functional, and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology. 1996 Jul;111(1):8–18. doi: 10.1053/gast.1996.v111.pm8698229. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Chung M., Gall D. G. Effect of intestinal anaphylaxis on gut function in the rat. Gastroenterology. 1984 Mar;86(3):391–397. [PubMed] [Google Scholar]
- Perdue M. H., Gall D. G. Intestinal anaphylaxis in the rat: jejunal response to in vitro antigen exposure. Am J Physiol. 1986 Apr;250(4 Pt 1):G427–G431. doi: 10.1152/ajpgi.1986.250.4.G427. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Masson S., Wershil B. K., Galli S. J. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991 Feb;87(2):687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H. C., Podolsky D. K. Human intestinal epithelial cells express functional cytokine receptors sharing the common gamma c chain of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8353–8357. doi: 10.1073/pnas.92.18.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. J., Welsh M. J. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992 Apr;89(4):1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. C., Matthews J., Andres P., Baffis V., Zheng X. X., Chae D. W., Smith J., Strom T. B., Maslinski W. Interleukin-15 signals T84 colonic epithelial cells in the absence of the interleukin-2 receptor beta-chain. Am J Physiol. 1997 May;272(5 Pt 1):G1201–G1208. doi: 10.1152/ajpgi.1997.272.5.G1201. [DOI] [PubMed] [Google Scholar]
- Yagita H., Nakata M., Azuma A., Nitta T., Takeshita T., Sugamura K., Okumura K. Activation of peripheral blood T cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Oct 1;170(4):1445–1450. doi: 10.1084/jem.170.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]