Abstract
BACKGROUND—Clonal T cell receptor (TCR) gene rearrangements and loss of T cell antigens such as CD8 and TCR-β in intraepithelial lymphocytes (IELs) may indicate the development of an enteropathy-type intestinal T cell lymphoma (EITCL) in patients with refractory sprue. AIMS—To define the diagnostic value of these markers in duodenal biopsies from patients with villous atrophy as a result of various underlying disorders. PATIENTS AND METHODS—Duodenal biopsies from eight patients with coeliac disease and five patients with villous atrophy caused by defined disorders were compared with three patients with refractory sprue evolving into overt EITCL, two patients with ulcerative jejunitis, and with eight patients with overt EITCL, for expression of CD3, CD4, CD8, and TCR-β in IELs using immunohistochemistry and for clonal TCR-γ gene rearrangements using polymerase chain reaction. In addition, biopsies from six consecutive patients with refractory sprue of uncertain cause were examined. RESULTS—Clonal TCR-γ gene rearrangements were found in all resected tumours of patients with EITCL, in 3/8 duodenal biopsies of patients with EITCL, in 2/2 patients with ulcerative jejunitis, in 2/3 patients with refractory sprue evolving into overt EITCL, and in 1/6 patients with refractory sprue. No rearrangements were found in biopsies from patients with refractory sprue caused by defined disorders or those with coeliac disease. Clonality in duodenal biopsies was associated with an abnormal phenotype of IELs in all cases and in all but one case in patients with evidence of underlying coeliac disease. Specificity for detection of an EITCL using immunohistology was 77% for CD8 and for TCR-β staining, and 100% for detection of a clonal TCR-γ gene rearrangement. Sensitivity was 62% for staining with CD8 and clonality investigation, while sensitivity reached 100% for TCR-β staining in all investigated patients with EITCL. CONCLUSIONS—Clonal proliferations of phenotypically abnormal IELs in refractory sprue represent an early manifestation of EITCL, for which the term "sprue-like intestinal T cell lymphoma" is proposed. This constellation is also found in duodenal biopsies from patients with an overt EITCL and is not related to other sprue syndromes, resulting in a high specificity for detection of an EITCL or refractory sprue evolving into EITCL. Overt EITCL may develop directly from coeliac disease without a precursor lesion (refractory sprue with clonal IELs) being demonstrable in duodenal biopsies or via a "sprue-like intestinal T cell lymphoma". This latter entity is a complication of coeliac disease. Keywords: T cell antigen; T cell receptor gene rearrangement; enteropathy-type intestinal T cell lymphoma; refractory sprue
Full Text
The Full Text of this article is available as a PDF (269.5 KB).
Figure 1 .
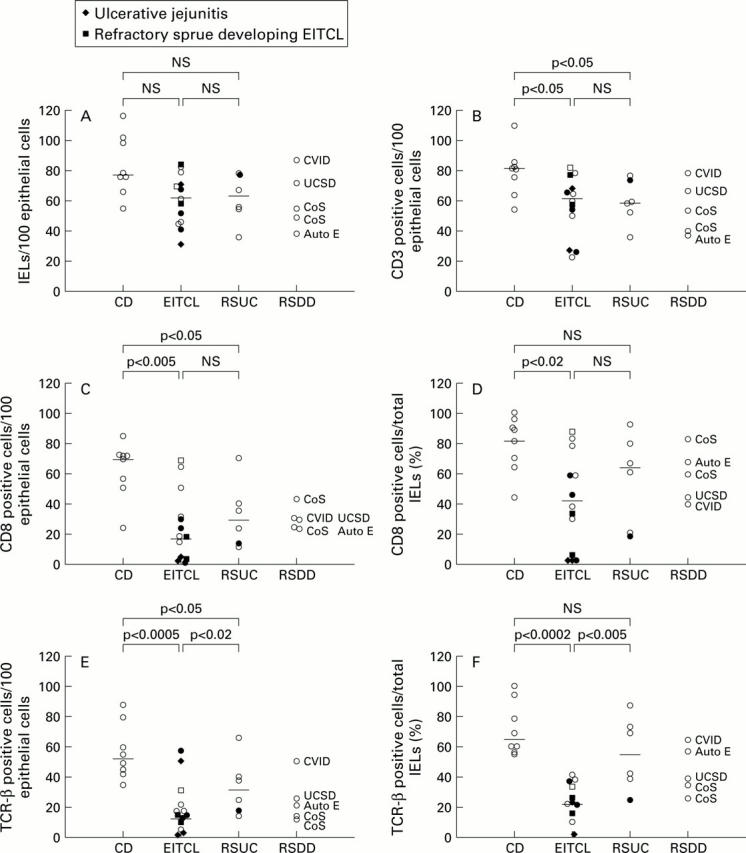
Absolute numbers of intraepithelial T lymphocytes (IELs) (A), and positively marked IELs with anti-CD3 (B), CD8 (C) and T cell receptor (TCR)-β (E) per 100 epithelial cells are shown. In addition, the percentage of positively marked IELs with anti-CD8 (D) and anti-TCR-β (F) of the total IELs was calculated. Clonal rearrangements of the TCR-γ gene are depicted by filled symbols. The number of CD 8 and TCR-β positive IELs in duodenal biopsies of patients with enteropathy-type intestinal T cell lymphomas (EITCLs) were significantly reduced in comparison with patients with coeliac disease (C-F). In most cases, clonality was associated with reduced expression of T cell antigens. RSUC, refractory sprue of uncertain cause; RSDD, refractory sprue due to defined disorders. For other abbreviations see table 1.
Figure 2 .
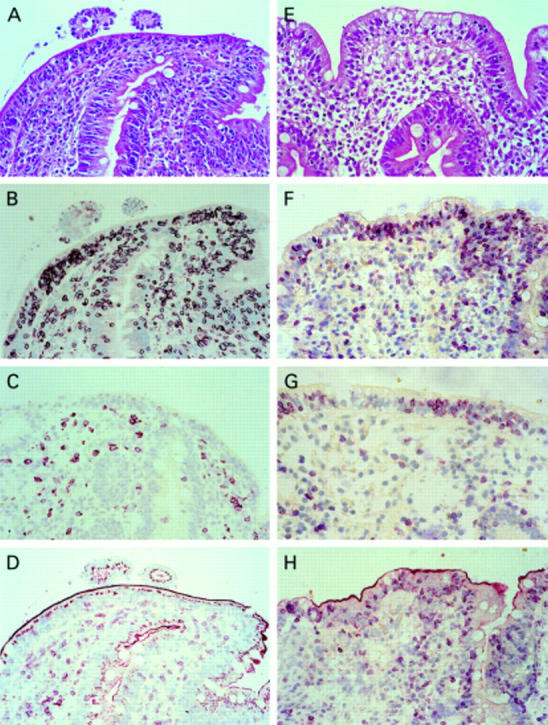
Haematoxylin-eosin staining (A) and immunohistochemistry (B-D) of a duodenal biopsy of a patient with an enteropathy-type intestinal T cell lymphoma (EITCL) showing an increased number of intraepithelial T lymphocytes (IELs) in conventional histology (A) that were mostly positive for CD3 (B) but negative for CD8 (C) and T cell receptor (TCR)-β (D), and a patient with coeliac disease (haematoxylin-eosin (E); CD3 (F); CD8 (G); TCR-β (H)). IELs in the duodenal biopsy of the patient with coeliac disease were CD3, CD8, and TCR-β positive (F-H). Original magnification 100×.
Figure 3 .
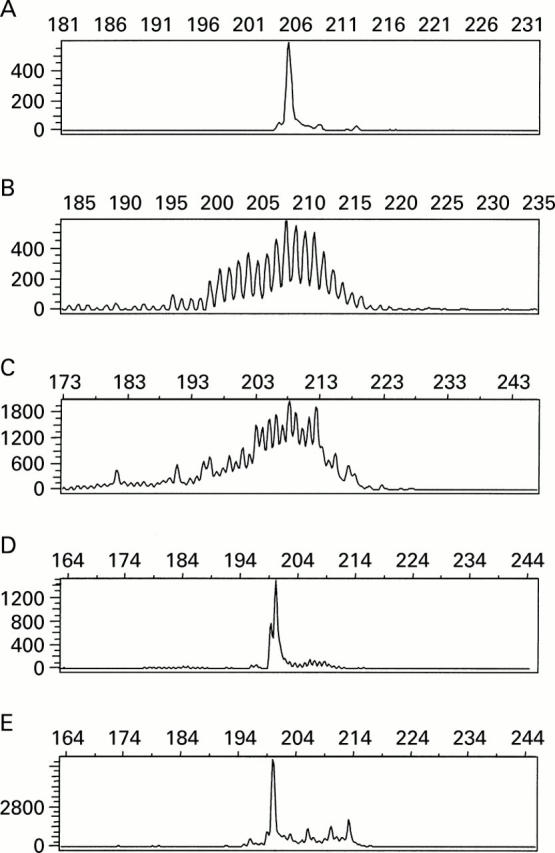
GeneScan analysis of polymerase chain reaction (PCR) products obtained by amplification of DNA from the T cell line PEER (A), of polyclonal tonsillar DNA (B), of DNA from a patient with coeliac disease showing polyclonal amplification (C), of DNA from a duodenal biopsy of patient EITCL7 with a reduced number of CD8 and T cell receptor (TCR) β positive intraepithelial T lymphocytes (IELs) on immunohistology (D), and of DNA from the lymphoma of the same patient showing the same length of monoclonal amplificate in both cases (E).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton-Key M., Diss T. C., Pan L., Du M. Q., Isaacson P. G. Molecular analysis of T-cell clonality in ulcerative jejunitis and enteropathy-associated T-cell lymphoma. Am J Pathol. 1997 Aug;151(2):493–498. [PMC free article] [PubMed] [Google Scholar]
- Bagdi E., Diss T. C., Munson P., Isaacson P. G. Mucosal intra-epithelial lymphocytes in enteropathy-associated T-cell lymphoma, ulcerative jejunitis, and refractory celiac disease constitute a neoplastic population. Blood. 1999 Jul 1;94(1):260–264. [PubMed] [Google Scholar]
- Bakels V., van Oostveen J. W., van der Putte S. C., Meijer C. J., Willemze R. Immunophenotyping and gene rearrangement analysis provide additional criteria to differentiate between cutaneous T-cell lymphomas and pseudo-T-cell lymphomas. Am J Pathol. 1997 Jun;150(6):1941–1949. [PMC free article] [PubMed] [Google Scholar]
- Brousse N., Verkarre V., Patey-Mariaud de Serre N., Cellier C., Cerf-Bensussan N., Delabesse E., Macintyre E. Is complicated celiac disease or refractory sprue an intestinal intra-epithelia cryptic T-cell lymphoma? Blood. 1999 May 1;93(9):3154–3155. [PubMed] [Google Scholar]
- Carbonnel F., Grollet-Bioul L., Brouet J. C., Teilhac M. F., Cosnes J., Angonin R., Deschaseaux M., Châtelet F. P., Gendre J. P., Sigaux F. Are complicated forms of celiac disease cryptic T-cell lymphomas? Blood. 1998 Nov 15;92(10):3879–3886. [PubMed] [Google Scholar]
- Cellier C., Patey N., Mauvieux L., Jabri B., Delabesse E., Cervoni J. P., Burtin M. L., Guy-Grand D., Bouhnik Y., Modigliani R. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998 Mar;114(3):471–481. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- Chott A., Dragosics B., Radaszkiewicz T. Peripheral T-cell lymphomas of the intestine. Am J Pathol. 1992 Dec;141(6):1361–1371. [PMC free article] [PubMed] [Google Scholar]
- Chott A., Haedicke W., Mosberger I., Födinger M., Winkler K., Mannhalter C., Müller-Hermelink H. K. Most CD56+ intestinal lymphomas are CD8+CD5-T-cell lymphomas of monomorphic small to medium size histology. Am J Pathol. 1998 Nov;153(5):1483–1490. doi: 10.1016/S0002-9440(10)65736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum S., Hummel M., Weiss D., Peters M., Wiedenmann B., Schäper F., Stein H., Riecken E. O., Foss H. Refractory sprue syndrome with clonal intraepithelial lymphocytes evolving into overt enteropathy-type intestinal T-cell lymphoma. Digestion. 2000;62(1):60–65. doi: 10.1159/000007779. [DOI] [PubMed] [Google Scholar]
- Dippel E., Assaf C., Hummel M., Schrag H. J., Stein H., Goerdt S., Orfanos C. E. Clonal T-cell receptor gamma-chain gene rearrangement by PCR-based GeneScan analysis in advanced cutaneous T-cell lymphoma: a critical evaluation. J Pathol. 1999 Jun;188(2):146–154. doi: 10.1002/(SICI)1096-9896(199906)188:2<146::AID-PATH334>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Domizio P., Owen R. A., Shepherd N. A., Talbot I. C., Norton A. J. Primary lymphoma of the small intestine. A clinicopathological study of 119 cases. Am J Surg Pathol. 1993 May;17(5):429–442. doi: 10.1097/00000478-199305000-00001. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Scott H., Brandtzaeg P. Intraepithelial T cells of the TcR gamma/delta+ CD8- and V delta 1/J delta 1+ phenotypes are increased in coeliac disease. Scand J Immunol. 1989 Dec;30(6):665–672. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Holmes G. K., Prior P., Lane M. R., Pope D., Allan R. N. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989 Mar;30(3):333–338. doi: 10.1136/gut.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. K., Stokes P. L., Sorahan T. M., Prior P., Waterhouse J. A., Cooke W. T. Coeliac disease, gluten-free diet, and malignancy. Gut. 1976 Aug;17(8):612–619. doi: 10.1136/gut.17.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson P. G. Gastrointestinal lymphoma. Hum Pathol. 1994 Oct;25(10):1020–1029. doi: 10.1016/0046-8177(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Murray A., Cuevas E. C., Jones D. B., Wright D. H. Study of the immunohistochemistry and T cell clonality of enteropathy-associated T cell lymphoma. Am J Pathol. 1995 Feb;146(2):509–519. [PMC free article] [PubMed] [Google Scholar]
- Pirisi-Hauck N. C., Foss H. D., Baier J., Kurunczi S. Simultaneous occurrence of autoimmune enteropathy and recurrent deep venous thrombosis. J Pediatr Gastroenterol Nutr. 2000 Mar;30(3):324–329. doi: 10.1097/00005176-200003000-00022. [DOI] [PubMed] [Google Scholar]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990 Aug;65(8):909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Gräff A., Hummel M., Zemlin M., Schneider T., Ullrich R., Heise W., Zeitz M., Riecken E. O., Stein H. Intestinal T-cell lymphoma: a reassessment of cytomorphological and phenotypic features in relation to patterns of small bowel remodelling. Virchows Arch. 1996 Sep;429(1):27–36. doi: 10.1007/BF00196817. [DOI] [PubMed] [Google Scholar]
- Spencer J., Cerf-Bensussan N., Jarry A., Brousse N., Guy-Grand D., Krajewski A. S., Isaacson P. G. Enteropathy-associated T cell lymphoma (malignant histiocytosis of the intestine) is recognized by a monoclonal antibody (HML-1) that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1988 Jul;132(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Spencer J., MacDonald T. T., Diss T. C., Walker-Smith J. A., Ciclitira P. J., Isaacson P. G. Changes in intraepithelial lymphocyte subpopulations in coeliac disease and enteropathy associated T cell lymphoma (malignant histiocytosis of the intestine). Gut. 1989 Mar;30(3):339–346. doi: 10.1136/gut.30.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H., Dienemann D., Sperling M., Zeitz M., Riecken E. O. Identification of a T cell lymphoma category derived from intestinal-mucosa-associated T cells. Lancet. 1988 Nov 5;2(8619):1053–1054. doi: 10.1016/S0140-6736(88)90068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier J. S. Diagnosis of celiac sprue. Gastroenterology. 1998 Jul;115(1):211–216. doi: 10.1016/s0016-5085(98)70383-x. [DOI] [PubMed] [Google Scholar]
- Trier J. S., Falchuk Z. M., Carey M. C., Schreiber D. S. Celiac sprue and refractory sprue. Gastroenterology. 1978 Aug;75(2):307–316. [PubMed] [Google Scholar]
- Volta U., Molinaro N., Fusconi M., Cassani F., Bianchi F. B. IgA antiendomysial antibody test. A step forward in celiac disease screening. Dig Dis Sci. 1991 Jun;36(6):752–756. doi: 10.1007/BF01311232. [DOI] [PubMed] [Google Scholar]
- Weinstein W. M., Saunders D. R., Tytgat G. N., Rubin C. E. Collagenous sprue--an unrecognized type of malabsorption. N Engl J Med. 1970 Dec 10;283(24):1297–1301. doi: 10.1056/NEJM197012102832401. [DOI] [PubMed] [Google Scholar]


