Abstract
BACKGROUND—Preascitic cirrhotic patients receiving 200 mmol of sodium daily for seven days remain in positive sodium balance. Thereafter, sodium handling is unknown. AIM—To assess renal sodium handling in preascitic cirrhosis on a high sodium diet for five weeks. METHODS—Sixteen biopsy proven preascitic cirrhotics were assessed at weekly intervals for five weeks on a diet of 200 mmol sodium/day using a daily weight diary and weekly 24 hour urinary sodium estimations. Fasting supine neurohormone levels were measured at baseline and weekly for five weeks while haemodynamics were measured at baseline and at five weeks. RESULTS—The daily diet of 200 mmol of sodium resulted in weight gain and a positive sodium balance for three weeks, associated with significant suppression of plasma renin activity and aldosterone levels, and a significant rise in plasma atrial natriuretic peptide levels (p<0.05). Patients' weights plateaued during week 4, associated with complete sodium balance and significant suppression of plasma noradrenaline levels (p<0.05). This was followed by a negative sodium balance and weight loss, and finally complete sodium balance, again despite a mean net gain of 2.3 (0.3) kg, associated with a return of plasma renin activity and aldosterone levels to within normal ranges. The lack of increase in central blood volume in addition to the persistent increase in plasma atrial natriuretic peptide levels indicated that residual volume expansion, consequent to persistent weight gain, was distributed on the venous side of the circulation. No free fluid was seen on repeat abdominal ultrasound after five weeks. CONCLUSION—Preascitic cirrhotics have a natriuretic "escape" after three weeks on high sodium dietary intake, associated with elevated plasma atrial natriuretic peptide levels and suppression of the renin-angiotensin-aldosterone system. With continued suppressed sympathetic activity, preascitics re-establish complete sodium balance but with a net weight gain and presumed increased intravascular volume, but without ascites. This further elucidates the compensated sodium retaining abnormality that characterises preascitic cirrhosis. Keywords: preascitic cirrhosis; sodium handling; renin-angiotensin-aldosterone system
Full Text
The Full Text of this article is available as a PDF (119.4 KB).
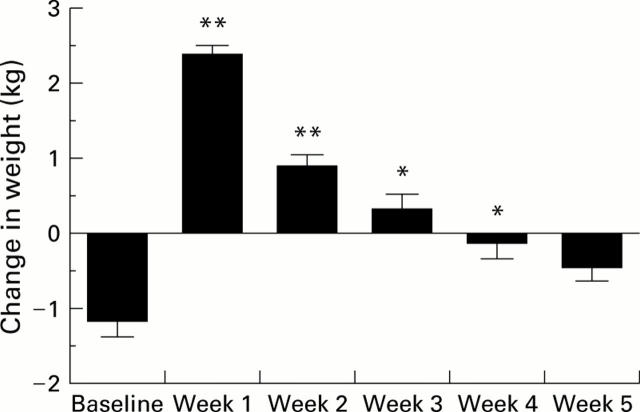
Figure 1 .
Weekly weight change in preascitic cirrhotic patients. *p<0.05, **p<0.01 compared with baseline.
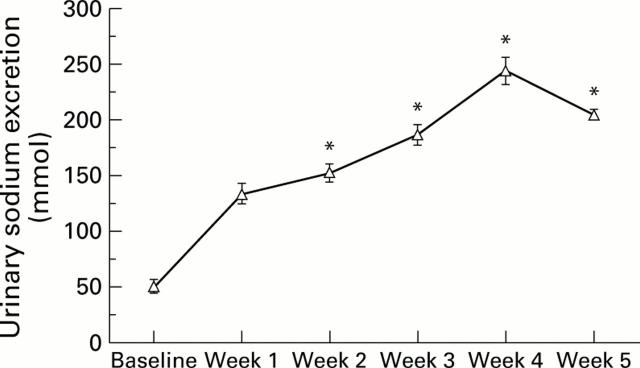
Figure 2 .
Urinary sodium excretion in preascitic cirrhotic patients. *p<0.05 compared with week 1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi M., Di Marco C., Trevisani F., Fornalè L., Andreone P., Cursaro C., Baraldini M., Ligabue A., Tamè M. R., Gasbarrini G. Renal sodium retention during upright posture in preascitic cirrhosis. Gastroenterology. 1993 Jul;105(1):188–193. doi: 10.1016/0016-5085(93)90025-8. [DOI] [PubMed] [Google Scholar]
- Bernardi M., Trevisani F., Gasbarrini A., Gasbarrini G. Hepatorenal disorders: role of the renin-angiotensin-aldosterone system. Semin Liver Dis. 1994 Feb;14(1):23–34. doi: 10.1055/s-2007-1007295. [DOI] [PubMed] [Google Scholar]
- Bernardi M., Trevisani F., Santini C., De Palma R., Gasbarrini G. Aldosterone related blood volume expansion in cirrhosis before and during the early phase of ascites formation. Gut. 1983 Aug;24(8):761–766. doi: 10.1136/gut.24.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M., Loutzenhiser R., Norsk P., Atlas S. Relationship between plasma ANF responsiveness and renal sodium handling in cirrhotic humans. Am J Nephrol. 1989;9(2):133–143. doi: 10.1159/000167952. [DOI] [PubMed] [Google Scholar]
- Eriksson B. M., Persson B. A. Determination of catecholamines in rat heart tissue and plasma samples by liquid chromatography with electrochemical detection. J Chromatogr. 1982 Mar 12;228:143–154. doi: 10.1016/s0378-4347(00)80427-2. [DOI] [PubMed] [Google Scholar]
- Genecin P., Polio J., Groszmann R. J. Na restriction blunts expansion of plasma volume and ameliorates hyperdynamic circulation in portal hypertension. Am J Physiol. 1990 Sep;259(3 Pt 1):G498–G503. doi: 10.1152/ajpgi.1990.259.3.G498. [DOI] [PubMed] [Google Scholar]
- Goodman J. M., McLaughlin P. R., Plyley M. J., Holloway R. M., Fell D., Logan A. G., Liu P. P. Impaired cardiopulmonary response to exercise in moderate hypertension. Can J Cardiol. 1992 May;8(4):363–371. [PubMed] [Google Scholar]
- Henriksen J. H., Bendtsen F., Sørensen T. I., Stadeager C., Ring-Larsen H. Reduced central blood volume in cirrhosis. Gastroenterology. 1989 Dec;97(6):1506–1513. doi: 10.1016/0016-5085(89)90396-x. [DOI] [PubMed] [Google Scholar]
- Iwao T., Oho K., Nakano R., Sakai T., Sato M., Miyamoto Y., Kumamoto M., Sakai K., Sata M., Toyonaga A. High plasma cardiac natriuretic peptides associated with enhanced cyclic guanosine monophosphate production in preascitic cirrhosis. J Hepatol. 2000 Mar;32(3):426–433. doi: 10.1016/s0168-8278(00)80393-1. [DOI] [PubMed] [Google Scholar]
- Katsuta Y., Aramaki T., Sekiyama T., Satomura K., Okumura H., Okumura K [corrected to Okumura H. ]. Plasma volume contraction in portal hypertension. J Hepatol. 1993;17 (Suppl 2):S19–S23. doi: 10.1016/s0168-8278(05)80450-7. [DOI] [PubMed] [Google Scholar]
- La Villa G., Lazzeri C., Pascale A., Sestini S., Bisi G., Sciagrà R., Vecchiarino S., Raggi V. C., Barletta G., Laffi G. Cardiovascular and renal effects of low-dose atrial natriuretic peptide in compensated cirrhosis. Am J Gastroenterol. 1997 May;92(5):852–857. [PubMed] [Google Scholar]
- La Villa G., Salmerón J. M., Arroyo V., Bosch J., Ginés P., García-Pagán J. C., Ginés A., Asbert M., Jiménez W., Rivera F. Mineralocorticoid escape in patients with compensated cirrhosis and portal hypertension. Gastroenterology. 1992 Jun;102(6):2114–2119. doi: 10.1016/0016-5085(92)90340-5. [DOI] [PubMed] [Google Scholar]
- Larose P., Meloche S., du Souich P., Deléan A., Ong H. Radioimmunoassay of atrial natriuretic factor: human plasma levels. Biochem Biophys Res Commun. 1985 Jul 31;130(2):553–558. doi: 10.1016/0006-291x(85)90452-8. [DOI] [PubMed] [Google Scholar]
- Møller S., Søndergaard L., Møgelvang J., Henriksen O., Henriksen J. H. Decreased right heart blood volume determined by magnetic resonance imaging: evidence of central underfilling in cirrhosis. Hepatology. 1995 Aug;22(2):472–478. doi: 10.1002/hep.1840220216. [DOI] [PubMed] [Google Scholar]
- Okumura H., Aramaki T., Katsuta Y., Satomura K., Akaike M., Sekiyama T., Terada H., Ohsuga M., Komeichi H., Tsutsui H. Reduction in hepatic venous pressure gradient as a consequence of volume contraction due to chronic administration of spironolactone in patients with cirrhosis and no ascites. Am J Gastroenterol. 1991 Jan;86(1):46–52. [PubMed] [Google Scholar]
- Sansoè G., Ferrari A., Baraldi E., Castellana C. N., De Santis M. C., Manenti F. Renal distal tubular handling of sodium in central fluid volume homoeostasis in preascitic cirrhosis. Gut. 1999 Nov;45(5):750–755. doi: 10.1136/gut.45.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani F., Bernardi M., De Palma R., Pancione L., Capani F., Baraldini M., Ligabue A., Gasbarrini G. Circadian variation in renal sodium and potassium handling in cirrhosis. The role of aldosterone, cortisol, sympathoadrenergic tone, and intratubular factors. Gastroenterology. 1989 Apr;96(4):1187–1198. doi: 10.1016/0016-5085(89)91640-5. [DOI] [PubMed] [Google Scholar]
- Trevisani F., Bernardi M., Gasbarrini A., Tamè M. R., Giancane S., Andreone P., Baraldini M., Cursaro C., Ligabue A., Gasbarrini G. Bed-rest-induced hypernatriuresis in cirrhotic patients without ascites: does it contribute to maintain 'compensation'? J Hepatol. 1992 Sep;16(1-2):190–196. doi: 10.1016/s0168-8278(05)80114-x. [DOI] [PubMed] [Google Scholar]
- Trevisani F., Colantoni A., Sica G., Gasbarrini A., D'Intino P. E., De Notariis S., De Jaso R., Barbieri A., Morselli A., Gasbarrini G. High plasma levels of atrial natriuretic peptide in preascitic cirrhosis: indirect evidence of reduced natriuretic effectiveness of the peptide. Hepatology. 1995 Jul;22(1):132–137. [PubMed] [Google Scholar]
- Ubeda M., Matzilevich M. M., Atucha N. M., García-Estañ J., Quesada T., Tang S. S., Ingelfinger J. R. Renin and angiotensinogen mRNA expression in the kidneys of rats subjected to long-term bile duct ligation. Hepatology. 1994 Jun;19(6):1431–1436. [PubMed] [Google Scholar]
- Warner L. C., Campbell P. J., Morali G. A., Logan A. G., Skorecki K. L., Blendis L. M. The response of atrial natriuretic factor and sodium excretion to dietary sodium challenges in patients with chronic liver disease. Hepatology. 1990 Sep;12(3 Pt 1):460–466. doi: 10.1002/hep.1840120303. [DOI] [PubMed] [Google Scholar]
- Weicker H., Feraudi M., Hägele H., Pluto R. Electrochemical detection of catecholamines in urine and plasma after separation with HPLC. Clin Chim Acta. 1984 Aug 15;141(1):17–25. doi: 10.1016/0009-8981(84)90162-1. [DOI] [PubMed] [Google Scholar]
- Wernze H., Spech H. J., Müller G. Studies on the activity of the renin-angiotensin-aldosterone system (RAAS) in patients with cirrhosis of the liver. Klin Wochenschr. 1978 Apr 15;56(8):389–397. doi: 10.1007/BF01477293. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. P., Smith I. K., Moodie H., Poston L., Williams R. Studies on mineralocorticoid 'escape' in cirrhosis. Clin Sci (Lond) 1979 May;56(5):401–406. doi: 10.1042/cs0560401. [DOI] [PubMed] [Google Scholar]
- Wong F., Blendis L. Pathophysiology of sodium retention and ascites formation in cirrhosis: role of atrial natriuretic factor. Semin Liver Dis. 1994 Feb;14(1):59–70. doi: 10.1055/s-2007-1007298. [DOI] [PubMed] [Google Scholar]
- Wong F., Liu P., Allidina Y., Blendis L. Pattern of sodium handling and its consequences in patients with preascitic cirrhosis. Gastroenterology. 1995 Jun;108(6):1820–1827. doi: 10.1016/0016-5085(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Wong F., Liu P., Tobe S., Morali G., Blendis L. Central blood volume in cirrhosis: measurement with radionuclide angiography. Hepatology. 1994 Feb;19(2):312–321. [PubMed] [Google Scholar]
- Wong F., Logan A., Blendis L. Systemic hemodynamic, forearm vascular, renal, and humoral responses to sustained cardiopulmonary baroreceptor deactivation in well-compensated cirrhosis. Hepatology. 1995 Mar;21(3):717–724. [PubMed] [Google Scholar]
- Wong F., Massie D., Hsu P., Dudley F. Renal response to a saline load in well-compensated alcoholic cirrhosis. Hepatology. 1994 Oct;20(4 Pt 1):873–881. doi: 10.1002/hep.1840200415. [DOI] [PubMed] [Google Scholar]
- Wong F., Sniderman K., Blendis L. The renal sympathetic and renin-angiotensin response to lower body negative pressure in well-compensated cirrhosis. Gastroenterology. 1998 Aug;115(2):397–405. doi: 10.1016/s0016-5085(98)70206-9. [DOI] [PubMed] [Google Scholar]