Abstract
BACKGROUND AND AIMS—Cytokeratin (CK) 7 and 20 patterns are specific for long and short segments of Barrett's oesophagus but their use has not been assessed in intestinal metaplasia arising in macroscopically normal gastro-oesophageal junction (GOJ). PATIENTS AND METHODS—This study was carried out in a large prospective series of 254 patients who underwent upper endoscopy, had normal gastro-oesophageal anatomy, and who had biopsies of the antrum, fundus, cardia, GOJ, and lower oesophagus. Intestinal metaplasia of the GOJ was typed by histochemistry with high iron diamine-alcian blue staining and by immunohistochemistry using CK7 and CK20 antibodies. Results were correlated with clinical, endoscopic, and pathological data. RESULTS—Sixty (23.6%) of our patients presenting with a normal GOJ had intestinal metaplasia. The CK7/CK20 pattern identified two groups of patients: one highly correlated with Barrett's and the other with characteristics of Helicobacter pylori gastritis. The Barrett's type CK7/CK20 pattern was related to a high frequency of gastro-oesophageal reflux symptoms (p<0.02) and normal endoscopic appearance of the stomach (p<0.03). In contrast, the gastric type CK7/CK20 pattern was linked to atrophic (p<0.02) or erythematous (p<0.05) appearance of the stomach (p<0.03), high frequency of H pylori infection (p<0.04), antral inflammation (p<0.006) with atrophy (p<0.02), and intestinal metaplasia (p<0.02). CONCLUSION—In patients presenting with intestinal metaplasia in normal appearing GOJ, the cytokeratin pattern identifies two groups of patients, one with features identical to those of long segment Barrett's oesophagus and one with features seen in H pylori gastritis. These data may be used by clinicians and should result in improved endoscopic surveillance strategies targeted specifically at patients at increased risk of Barrett's oesophagus and thus cancer. Keywords: Barrett's oesophagus; cardia; intestinal metaplasia; cytokeratins
Full Text
The Full Text of this article is available as a PDF (216.4 KB).
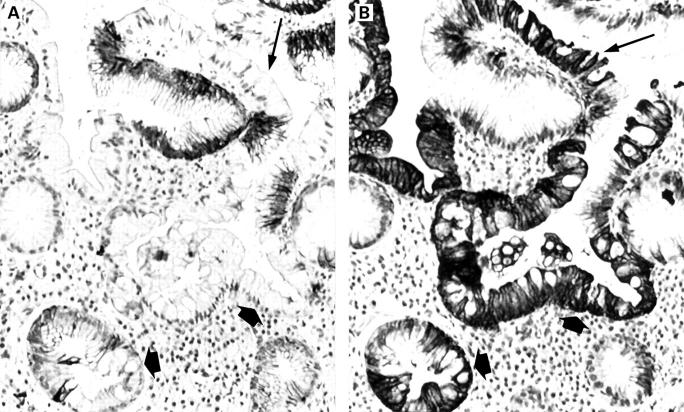
Figure 1 .
Barrett's cytokeratin 7 and 20 (CK7/CK20) pattern in biopsy specimens from the Z line with incomplete (A) and complete (B) intestinal metaplasia. (A) Diffuse strong CK7 staining of surface epithelium (arrow) and deep glands (arrowheads). Immunoperoxidase with nuclear counterstain by Mayer's haematoxylin (original magnification ×200). (B) Band-like CK20 staining of superficial epithelium (arrow). Absent immunoreactivity of deep glands (arrowheads). Immunoperoxidase with nuclear counterstain by Mayer's haematoxylin (original magnification ×200).
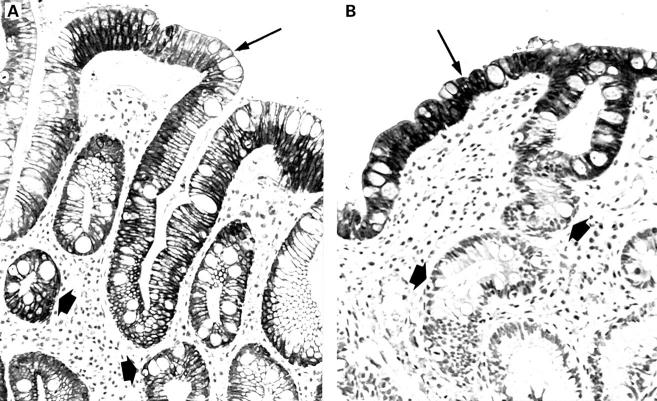
Figure 2 .
Gastric cytokeratin 7 and 20 (CK7/CK20) pattern in a biopsy specimen from the Z line with complete intestinal metaplasia. (A) Absent CK7 staining of superficial epithelium (arrow) and deep glands (arrowheads). Immunoperoxidase with nuclear counterstain by Mayer's haematoxylin (original magnification ×200). (B) Strong diffuse CK20 immunostaining of both superficial epithelium (arrow) and deep glands (arrowheads). Immunoperoxidase with nuclear counterstain by Mayer's haematoxylin (original magnification ×200).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blot W. J., Devesa S. S., Kneller R. W., Fraumeni J. F., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991 Mar 13;265(10):1287–1289. [PubMed] [Google Scholar]
- Burnett R. A., Brown I. L., Findlay J. Cresyl fast violet staining method for Campylobacter like organisms. J Clin Pathol. 1987 Mar;40(3):353–353. doi: 10.1136/jcp.40.3.353-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. J., Lomboy C. T. Barrett's esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology. 1992 Oct;103(4):1241–1245. doi: 10.1016/0016-5085(92)91510-b. [DOI] [PubMed] [Google Scholar]
- Chen Y. Y., Antonioli D. A., Spechler S. J., Zeroogian J. M., Goyal R. K., Wang H. H. Gastroesophageal reflux disease versus Helicobacter pylori infection as the cause of gastric carditis. Mod Pathol. 1998 Oct;11(10):950–956. [PubMed] [Google Scholar]
- Csendes A., Smok G., Burdiles P., Quesada F., Huertas C., Rojas J., Korn O. Prevalence of Barrett's esophagus by endoscopy and histologic studies: a prospective evaluation of 306 control subjects and 376 patients with symptoms of gastroesophageal reflux. Dis Esophagus. 2000;13(1):5–11. doi: 10.1046/j.1442-2050.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- Csendes A., Smok G., Burdiles P., Sagastume H., Rojas J., Puente G., Quezada F., Korn O. 'Carditis': an objective histological marker for pathologic gastroesophageal reflux disease. Dis Esophagus. 1998 Apr;11(2):101–105. doi: 10.1093/dote/11.2.101. [DOI] [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Goldstein N. S. Gastric cardia intestinal metaplasia: biopsy follow-up of 85 patients. Mod Pathol. 2000 Oct;13(10):1072–1079. doi: 10.1038/modpathol.3880198. [DOI] [PubMed] [Google Scholar]
- Goldstein N. S., Karim R. Gastric cardia inflammation and intestinal metaplasia: associations with reflux esophagitis and Helicobacter pylori. Mod Pathol. 1999 Nov;12(11):1017–1024. [PubMed] [Google Scholar]
- Günther T., Hackelsberger A., Malfertheiner P., Roessner A. Is typing of metaplasia at the squamocolumnar junction revealing its aetiology? Virchows Arch. 2000 Jan;436(1):6–11. doi: 10.1007/pl00008200. [DOI] [PubMed] [Google Scholar]
- Hackelsberger A., Günther T., Schultze V., Manes G., Dominguez-Muñoz J. E., Roessner A., Malfertheiner P. Intestinal metaplasia at the gastro-oesophageal junction: Helicobacter pylori gastritis or gastro-oesophageal reflux disease? Gut. 1998 Jul;43(1):17–21. doi: 10.1136/gut.43.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggitt R. C. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994 Oct;25(10):982–993. doi: 10.1016/0046-8177(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Haggitt R. C., Reid B. J., Rabinovitch P. S., Rubin C. E. Barrett's esophagus. Correlation between mucin histochemistry, flow cytometry, and histologic diagnosis for predicting increased cancer risk. Am J Pathol. 1988 Apr;131(1):53–61. [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. R., Smith R. R. The relationship between columnar epithelial dysplasia and invasive adenocarcinoma arising in Barrett's esophagus. Am J Clin Pathol. 1987 Mar;87(3):301–312. doi: 10.1093/ajcp/87.3.301. [DOI] [PubMed] [Google Scholar]
- Jass J. R. Mucin histochemistry of the columnar epithelium of the oesophagus: a retrospective study. J Clin Pathol. 1981 Aug;34(8):866–870. doi: 10.1136/jcp.34.8.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapertosa G., Baracchini P., Fulcheri E. Mucin histochemical analysis in the interpretation of Barrett's esophagus. Results of a multicenter study. The Operative Group for the Study of Esophageal Precancer. Am J Clin Pathol. 1992 Jul;98(1):61–66. doi: 10.1093/ajcp/98.1.61. [DOI] [PubMed] [Google Scholar]
- Ormsby A. H., Goldblum J. R., Rice T. W., Richter J. E., Falk G. W., Vaezi M. F., Gramlich T. L. Cytokeratin subsets can reliably distinguish Barrett's esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999 Mar;30(3):288–294. doi: 10.1016/s0046-8177(99)90007-2. [DOI] [PubMed] [Google Scholar]
- Ormsby A. H., Vaezi M. F., Richter J. E., Goldblum J. R., Rice T. W., Falk G. W., Gramlich T. L. Cytokeratin immunoreactivity patterns in the diagnosis of short-segment Barrett's esophagus. Gastroenterology. 2000 Sep;119(3):683–690. doi: 10.1053/gast.2000.16482. [DOI] [PubMed] [Google Scholar]
- Pera M., Cameron A. J., Trastek V. F., Carpenter H. A., Zinsmeister A. R. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993 Feb;104(2):510–513. doi: 10.1016/0016-5085(93)90420-h. [DOI] [PubMed] [Google Scholar]
- Pereira A. D., Suspiro A., Chaves P., Saraiva A., Glória L., de Almeida J. C., Leitão C. N., Soares J., Mira F. C. Short segments of Barrett's epithelium and intestinal metaplasia in normal appearing oesophagogastric junctions: the same or two different entities? Gut. 1998 May;42(5):659–662. doi: 10.1136/gut.42.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuchmaur M., Potet F., Goldfain D. Mucin histochemistry of the columnar epithelium of the oesophagus (Barrett's oesophagus): a prospective biopsy study. J Clin Pathol. 1984 Jun;37(6):607–610. doi: 10.1136/jcp.37.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Sipponen P., Seppälä K., Varis K., Hjelt L., Ihamäki T., Kekki M., Siurala M. Intestinal metaplasia with colonic-type sulphomucins in the gastric mucosa; its association with gastric carcinoma. Acta Pathol Microbiol Scand A. 1980 Jul;88(4):217–224. doi: 10.1111/j.1699-0463.1980.tb02489.x. [DOI] [PubMed] [Google Scholar]
- Spechler S. J., Robbins A. H., Rubins H. B., Vincent M. E., Heeren T., Doos W. G., Colton T., Schimmel E. M. Adenocarcinoma and Barrett's esophagus. An overrated risk? Gastroenterology. 1984 Oct;87(4):927–933. [PubMed] [Google Scholar]
- Spechler S. J., Zeroogian J. M., Antonioli D. A., Wang H. H., Goyal R. K. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994 Dec 3;344(8936):1533–1536. doi: 10.1016/s0140-6736(94)90349-2. [DOI] [PubMed] [Google Scholar]
- Torrado J., Ruiz B., Garay J., Asenjo J. L., Tovar J. A., Cosme A., Correa P. Blood-group phenotypes, sulfomucins, and Helicobacter pylori in Barrett's esophagus. Am J Surg Pathol. 1997 Sep;21(9):1023–1029. doi: 10.1097/00000478-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Trudgill N. J., Suvarna S. K., Kapur K. C., Riley S. A. Intestinal metaplasia at the squamocolumnar junction in patients attending for diagnostic gastroscopy. Gut. 1997 Nov;41(5):585–589. doi: 10.1136/gut.41.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen M., Färkkilä M., Juhola M., Mecklin J. P., Sipponen P. Complete and incomplete intestinal metaplasia at the oesophagogastric junction: prevalences and associations with endoscopic erosive oesophagitis and gastritis. Gut. 1999 Nov;45(5):644–648. doi: 10.1136/gut.45.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen M., Färkkilä M., Mecklin J. P., Juhola M., Sipponen P. Classical Barrett esophagus contrasted with Barrett-type epithelium at normal-appearing esophagogastric junction. Central Finland Endoscopy Study Group. Scand J Gastroenterol. 2000 Jan;35(1):2–9. doi: 10.1080/003655200750024452. [DOI] [PubMed] [Google Scholar]