Abstract
BACKGROUND—Crohn's disease is a heterogeneous disorder with both a genetic and environmental aetiology. Clinical classifications of the disease, such as the newly proposed Vienna classification, may help to define subgroups of patients suitable for studying the influence of specific genetic or environmental factors. AIM—To assess the stability over the course of the disease of its location and behaviour, as determined according to the Vienna classification. PATIENTS AND METHODS—The notes of 297 Crohn's disease patients regularly followed up at our institution were carefully reviewed retrospectively. The behaviour and location of the disease according to the Vienna classification were determined at diagnosis and after 1, 3, 5, 10, 15, 20, and 25 years of follow up. The proportions of the different behaviours and locations of the disease were calculated at these time points. A statistical analysis of the evolution of these characteristics over 10 years was performed on a subgroup of 125 patients with at least 10 years of follow up. The influence of age at diagnosis on location and behaviour of the disease was assessed as well as the influence of location on the behaviour of the disease. RESULTS—The location of the disease remained relatively stable over the course of the disease. Although the proportion of patients who had a change in disease location became statistically significant after five years (p=0.01), over 10 years only 15.9% of patients had a change in location (p<0.001). We observed a more rapid and prominent change in disease behaviour, which was already statistically significant after one year (p=0.04). Over 10 years, 45.9% of patients had a change in disease behaviour (p<0.0001). The most prominent change was from non-stricturing non-penetrating disease to either stricturing (27.1%; p<0.0001) or penetrating (29.4%; p<0.0001) disease. Age at diagnosis had no influence on either location or behaviour of disease. Ileal Crohn's disease was more often stricturing, and colonic or ileocolonic Crohn's disease was more often penetrating: this was already the case at diagnosis and became more prominent after 10 years (p<0.05). CONCLUSIONS—Location of Crohn's disease, as defined by the Vienna classification, is a relatively stable phenotype which seems suitable for phenotype-genotype analyses. Behaviour of Crohn's disease according to the Vienna classification varies dramatically over the course of the disease and cannot be used in phenotype-genotype analyses. The potential influence of genes on the behaviour of Crohn's disease should be studied in subgroups of patients defined by their disease behaviour after a fixed duration of disease. Keywords: Crohn's disease; genetics; Vienna classification
Full Text
The Full Text of this article is available as a PDF (125.5 KB).
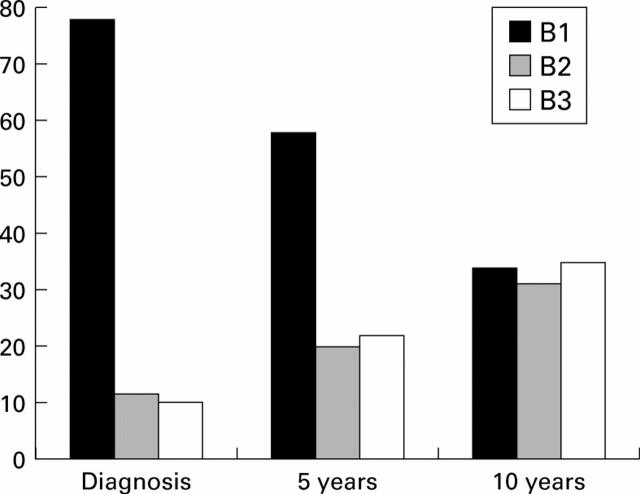
Figure 1 .
Evolution of disease behaviour according to the Vienna classification system over 10 years in 125 patients with Crohn's disease. The proportion of patients with non-stricturing non-penetrating (B1), stricturing (B2), and penetrating (B3) disease are shown at diagnosis, and after five and 10 years of evolution. The proportion of patients with a change in disease behaviour was highly significant over 10 years (p<0.0001) and was already significantly different from zero after one year (p=0.04).
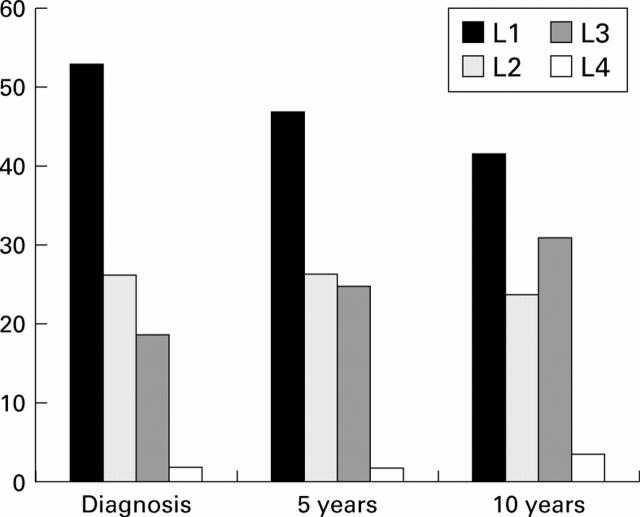
Figure 2 .
Evolution of disease location according to the Vienna classification system over 10 years in 125 patients with Crohn's disease. The proportion of patients with ileal (L1), colonic (L2), ileocolonic (L3), and upper gastrointestinal (L4) disease are shown at diagnosis, and after five and 10 years of evolution. The proportions of patients with a change in disease location was statistically significant over 10 years (p<0.001). This proportion became significantly different from zero after five years (p=0.01).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeberhard P., Berchtold W., Riedtmann H. J., Stadelmann G. Surgical recurrence of perforating and nonperforating Crohn's disease. A study of 101 surgically treated Patients. Dis Colon Rectum. 1996 Jan;39(1):80–87. doi: 10.1007/BF02048274. [DOI] [PubMed] [Google Scholar]
- Bayless T. M., Tokayer A. Z., Polito J. M., 2nd, Quaskey S. A., Mellits E. D., Harris M. L. Crohn's disease: concordance for site and clinical type in affected family members--potential hereditary influences. Gastroenterology. 1996 Sep;111(3):573–579. doi: 10.1053/gast.1996.v111.pm8780559. [DOI] [PubMed] [Google Scholar]
- Bouma G., Poen A. C., García-González M. A., Schreuder G. M., Felt-Bersma R. J., Meuwissen S. G., Pena A. S. HLA-DRB1*03, but not the TNFA -308 promoter gene polymorphism, confers protection against fistulising Crohn's disease. Immunogenetics. 1998 May;47(6):451–455. doi: 10.1007/s002510050382. [DOI] [PubMed] [Google Scholar]
- Farmer R. G., Hawk W. A., Turnbull R. B., Jr Indications for surgery in Crohn's disease: analysis of 500 cases. Gastroenterology. 1976 Aug;71(2):245–250. [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998 Jul;115(1):182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Gasche C., Scholmerich J., Brynskov J., D'Haens G., Hanauer S. B., Irvine E. J., Jewell D. P., Rachmilewitz D., Sachar D. B., Sandborn W. J. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000 Feb;6(1):8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Gilberts E. C., Greenstein A. J., Katsel P., Harpaz N., Greenstein R. J. Molecular evidence for two forms of Crohn disease. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12721–12724. doi: 10.1073/pnas.91.26.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein A. J., Lachman P., Sachar D. B., Springhorn J., Heimann T., Janowitz H. D., Aufses A. H., Jr Perforating and non-perforating indications for repeated operations in Crohn's disease: evidence for two clinical forms. Gut. 1988 May;29(5):588–592. doi: 10.1136/gut.29.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresbach D., Alizadeh M., Bretagne J. F., Dabadie A., Colombel J. F., Pagenault M., Heresbach-Le Berre N., Genetet B., Gosselin M., Semana G. TAP gene transporter polymorphism in inflammatory bowel diseases. Scand J Gastroenterol. 1997 Oct;32(10):1022–1027. doi: 10.3109/00365529709011219. [DOI] [PubMed] [Google Scholar]
- Hugot J. P., Chamaillard M., Zouali H., Lesage S., Cézard J. P., Belaiche J., Almer S., Tysk C., O'Morain C. A., Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001 May 31;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Lautenbach E., Berlin J. A., Lichtenstein G. R. Risk factors for early postoperative recurrence of Crohn's disease. Gastroenterology. 1998 Aug;115(2):259–267. doi: 10.1016/s0016-5085(98)70191-x. [DOI] [PubMed] [Google Scholar]
- Louis E., Peeters M., Franchimont D., Seidel L., Fontaine F., Demolin G., Croes F., Dupont P., Davin L., Omri S. Tumour necrosis factor (TNF) gene polymorphism in Crohn's disease (CD): influence on disease behaviour? Clin Exp Immunol. 2000 Jan;119(1):64–68. doi: 10.1046/j.1365-2249.2000.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y., Bonen D. K., Inohara N., Nicolae D. L., Chen F. F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R. H. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001 May 31;411(6837):603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Peeters M., Nevens H., Baert F., Hiele M., de Meyer A. M., Vlietinck R., Rutgeerts P. Familial aggregation in Crohn's disease: increased age-adjusted risk and concordance in clinical characteristics. Gastroenterology. 1996 Sep;111(3):597–603. doi: 10.1053/gast.1996.v111.pm8780562. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Grootscholten C., Holt H., Jewell D. P. Clinical patterns of familial inflammatory bowel disease. Gut. 1996 May;38(5):738–741. doi: 10.1136/gut.38.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsangi J., Landers C. J., Welsh K. I., Koss K., Targan S., Jewell D. P. The presence of anti-neutrophil antibodies reflects clinical and genetic heterogeneity within inflammatory bowel disease. Inflamm Bowel Dis. 1998 Feb;4(1):18–26. doi: 10.1097/00054725-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Steinhart A. H., Girgrah N., McLeod R. S. Reliability of a Crohn's disease clinical classification scheme based on disease behavior. Inflamm Bowel Dis. 1998 Aug;4(3):228–234. doi: 10.1097/00054725-199808000-00006. [DOI] [PubMed] [Google Scholar]
- Vasiliauskas E. A., Plevy S. E., Landers C. J., Binder S. W., Ferguson D. M., Yang H., Rotter J. I., Vidrich A., Targan S. R. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn's disease define a clinical subgroup. Gastroenterology. 1996 Jun;110(6):1810–1819. doi: 10.1053/gast.1996.v110.pm8964407. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Allan R. N., Keighley M. R. Perforating ileocecal Crohn's disease does not carry a high risk of recurrence but usually re-presents as perforating disease. Dis Colon Rectum. 1999 Apr;42(4):519–524. doi: 10.1007/BF02234180. [DOI] [PubMed] [Google Scholar]