Abstract
Objective—To describe the morphology of the pulmonary arteries in patients with pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries with and without monosomy 22q11. Design—A retrospective analysis of all patients with this congenital heart defect who are being followed at the University Children's Hospital Erlangen. Setting—A tertiary referral centre for paediatric cardiology and paediatric cardiac surgery. Patients—21 patients with pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries. Monosomy 22q11 was diagnosed by fluorescent in situ hybridisation using the D22S75 probe (Oncor). The morphology of the pulmonary arteries was assessed on the basis of selective angiograms. Results—10 patients (48%) were shown to have a microdeletion in 22q11 (group I). There was no difference with respect to the presence of confluent central pulmonary arteries between these patients (80%) and the remaining 11 patients (group II) without monosomy 22q11 (91%). Patients of group I, however, more often had arborisation anomalies of the pulmonary vascular bed (90% in group I v 27% in group II). Because of the more severe abnormalities of the pulmonary arteries, a biventricular repair had not been possible in any of the children with monosomy 22q11, though repair had been carried out in 64% of the children in group II. Conclusions—The developmental disturbance caused by monosomy 22q11 seems to impair the connection of the peripheral pulmonary artery segments to the central pulmonary arteries in patients with pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries, resulting in a lower probability of biventricular repair. Keywords: CATCH 22; pulmonary atresia and ventricular septal defect; major aortopulmonary collateral arteries; conotruncal anomaly face syndrome
Full Text
The Full Text of this article is available as a PDF (127.2 KB).
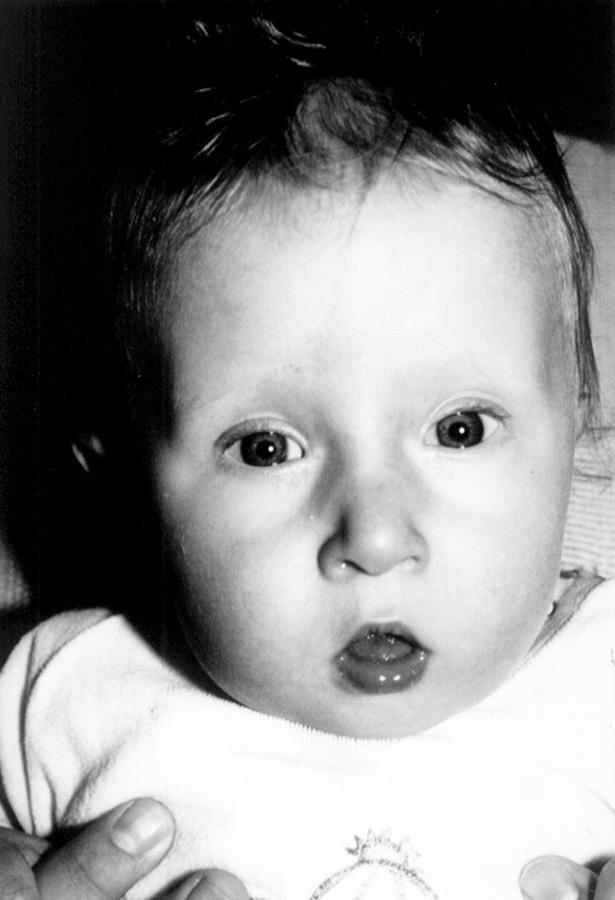
Figure 1 .
Conotruncal anomaly facies in patient 3.
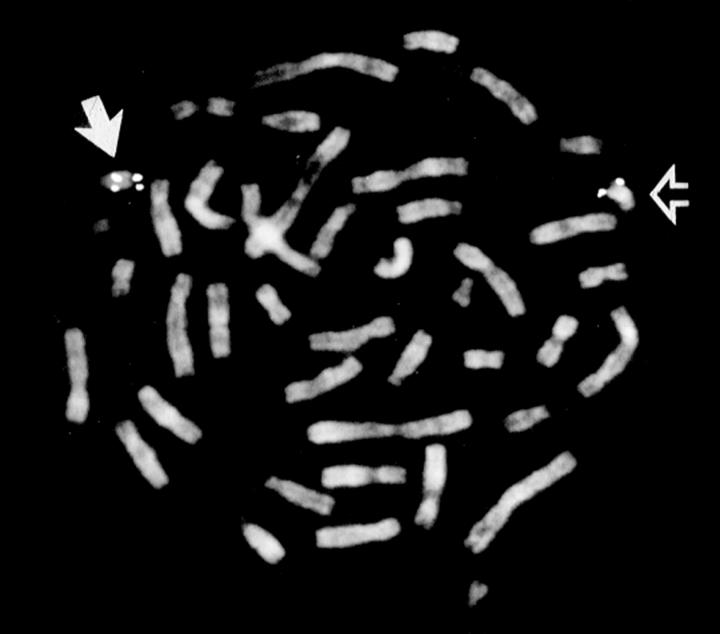
Figure 2 .
Metaphase spread after FISH with D22S75 probe in patient 5. The normal chromosome 22 has four fluorescein signals (arrow) representing the control (D22S39) and CATCH 22 regions (D22S75) on both chromatids. The chromosome 22 with a deletion has only two signals (open arrow) representing the control probe, but absent signals at 22q11.
Figure 3 .
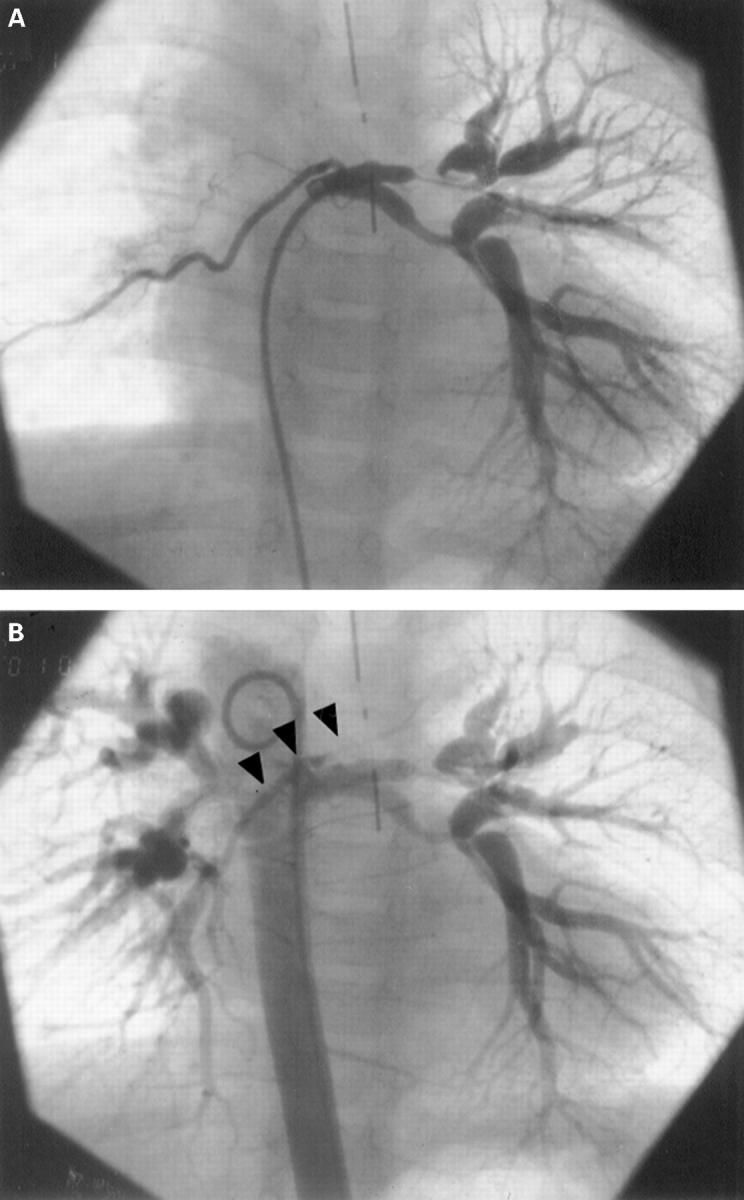
Selective injection into a major aortopulmonary collateral artery shows severe intrapulmonary stenoses of left pulmonary artery segments in a patient with monosomy 22q11 (A). Late frames following injection in the descending aorta (B) reveal the presence of severely hypoplastic central pulmonary arteries (arrows).
Figure 4 .
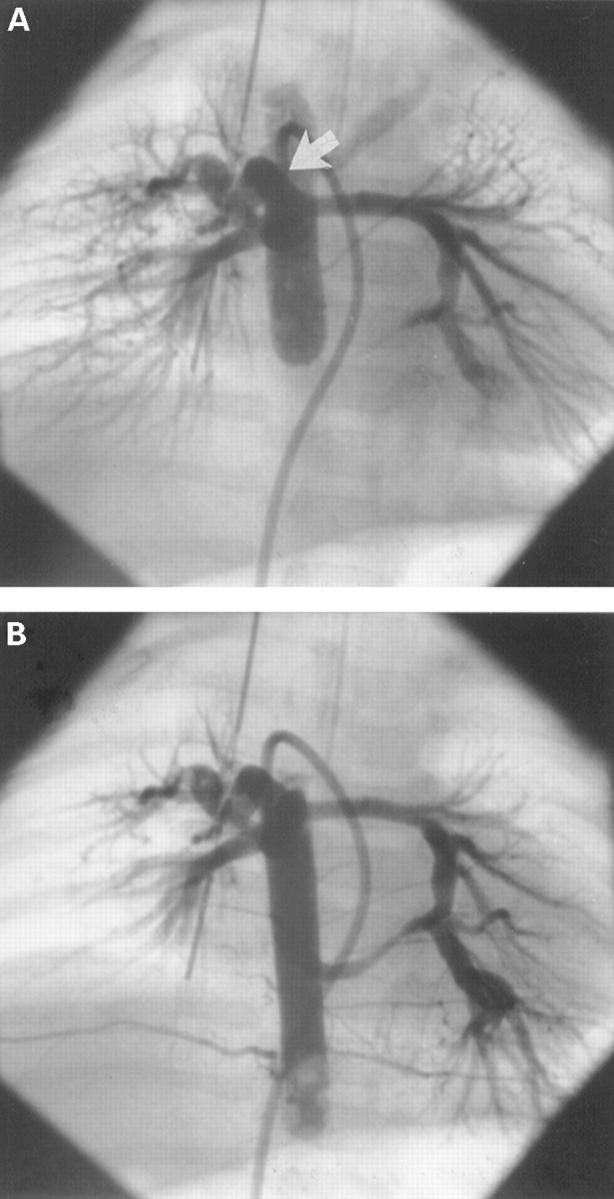
Unifocal pulmonary blood supply in a patient of group II (A). Balloon blockade angiography in the descending aorta shows filling of the central pulmonary arteries through a large major aortopulmonary collateral artery (arrow). Angiography at a lower level (B) reveals a second major aortopulmonary collateral connecting to the left pulmonary artery, with retrograde filling of the bifurcation (dual blood supply of the central pulmonary arteries).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Digilio M. C., Marino B., Grazioli S., Agostino D., Giannotti A., Dallapiccola B. Comparison of occurrence of genetic syndromes in ventricular septal defect with pulmonic stenosis (classic tetralogy of Fallot) versus ventricular septal defect with pulmonic atresia. Am J Cardiol. 1996 Jun 15;77(15):1375–1376. doi: 10.1016/s0002-9149(96)00212-3. [DOI] [PubMed] [Google Scholar]
- Dinarevic S., Redington A., Rigby M., Shinebourne E. A. Outcome of pulmonary atresia and ventricular septal defect during infancy. Pediatr Cardiol. 1995 Nov-Dec;16(6):276–282. doi: 10.1007/BF00798061. [DOI] [PubMed] [Google Scholar]
- Fäller K., Haworth S. G., Taylor J. F., Macartney F. J. Duplicate sources of pulmonary blood supply in pulmonary atresia with ventricular septal defect. Br Heart J. 1981 Sep;46(3):263–268. doi: 10.1136/hrt.46.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbeck M., Sunnegårdh J. T., Burrows P. E., Moes C. A., Lightfoot N., Williams W. G., Trusler G. A., Freedom R. M. Analysis of survival in patients with pulmonic valve atresia and ventricular septal defect. Am J Cardiol. 1991 Apr 1;67(8):737–743. doi: 10.1016/0002-9149(91)90532-p. [DOI] [PubMed] [Google Scholar]
- Jedele K. B., Michels V. V., Puga F. J., Feldt R. H. Velo-cardio-facial syndrome associated with ventricular septal defect, pulmonary atresia, and hypoplastic pulmonary arteries. Pediatrics. 1992 May;89(5 Pt 1):915–919. [PubMed] [Google Scholar]
- Kelly D., Goldberg R., Wilson D., Lindsay E., Carey A., Goodship J., Burn J., Cross I., Shprintzen R. J., Scambler P. J. Confirmation that the velo-cardio-facial syndrome is associated with haplo-insufficiency of genes at chromosome 22q11. Am J Med Genet. 1993 Feb 1;45(3):308–312. doi: 10.1002/ajmg.1320450306. [DOI] [PubMed] [Google Scholar]
- Kirby M. L., Waldo K. L. Role of neural crest in congenital heart disease. Circulation. 1990 Aug;82(2):332–340. doi: 10.1161/01.cir.82.2.332. [DOI] [PubMed] [Google Scholar]
- Macartney F. J., Scott O., Deverall P. B. Haemodynamic and anatomical characteristics of pulmonary blood supply in pulmonary atresia with ventricular septal defect - including a case of persistent fifth aortic arch. Br Heart J. 1974 Nov;36(11):1049–1060. doi: 10.1136/hrt.36.11.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino B., Digilio M. C., Grazioli S., Formigari R., Mingarelli R., Giannotti A., Dallapiccola B. Associated cardiac anomalies in isolated and syndromic patients with tetralogy of Fallot. Am J Cardiol. 1996 Mar 1;77(7):505–508. doi: 10.1016/s0002-9149(97)89345-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka R., Takao A., Kimura M., Imamura S., Kondo C., Joh-o K., Ikeda K., Nishibatake M., Ando M., Momma K. Confirmation that the conotruncal anomaly face syndrome is associated with a deletion within 22q11.2. Am J Med Genet. 1994 Nov 15;53(3):285–289. doi: 10.1002/ajmg.1320530314. [DOI] [PubMed] [Google Scholar]
- Momma K., Kondo C., Ando M., Matsuoka R., Takao A. Tetralogy of Fallot associated with chromosome 22q11 deletion. Am J Cardiol. 1995 Sep 15;76(8):618–621. doi: 10.1016/s0002-9149(99)80170-2. [DOI] [PubMed] [Google Scholar]
- Momma K., Kondo C., Matsuoka R., Takao A. Cardiac anomalies associated with a chromosome 22q11 deletion in patients with conotruncal anomaly face syndrome. Am J Cardiol. 1996 Sep 1;78(5):591–594. doi: 10.1016/s0002-9149(96)00374-8. [DOI] [PubMed] [Google Scholar]
- Momma K., Kondo C., Matsuoka R. Tetralogy of Fallot with pulmonary atresia associated with chromosome 22q11 deletion. J Am Coll Cardiol. 1996 Jan;27(1):198–202. doi: 10.1016/0735-1097(95)00415-7. [DOI] [PubMed] [Google Scholar]
- Puga F. J., Leoni F. E., Julsrud P. R., Mair D. D. Complete repair of pulmonary atresia, ventricular septal defect, and severe peripheral arborization abnormalities of the central pulmonary arteries. Experience with preliminary unifocalization procedures in 38 patients. J Thorac Cardiovasc Surg. 1989 Dec;98(6):1018–1029. [PubMed] [Google Scholar]
- Sawatari K., Imai Y., Kurosawa H., Isomatsu Y., Momma K. Staged operation for pulmonary atresia and ventricular septal defect with major aortopulmonary collateral arteries. New technique for complete unifocalization. J Thorac Cardiovasc Surg. 1989 Nov;98(5 Pt 1):738–750. [PubMed] [Google Scholar]
- Shimazaki Y., Iio M., Nakano S., Morimoto S., Ikawa S., Matsuda H., Kawashima Y. Pulmonary artery morphology and hemodynamics in pulmonic valve atresia with ventricular septal defect before and after repair. Am J Cardiol. 1991 Apr 1;67(8):744–748. doi: 10.1016/0002-9149(91)90533-q. [DOI] [PubMed] [Google Scholar]
- Shimazaki Y., Maehara T., Blackstone E. H., Kirklin J. W., Bargeron L. M., Jr The structure of the pulmonary circulation in tetralogy of Fallot with pulmonary atresia. A quantitative cineangiographic study. J Thorac Cardiovasc Surg. 1988 Jun;95(6):1048–1058. [PubMed] [Google Scholar]
- Van Mierop L. H., Kutsche L. M. Cardiovascular anomalies in DiGeorge syndrome and importance of neural crest as a possible pathogenetic factor. Am J Cardiol. 1986 Jul 1;58(1):133–137. doi: 10.1016/0002-9149(86)90256-0. [DOI] [PubMed] [Google Scholar]
- Wilson D. I., Burn J., Scambler P., Goodship J. DiGeorge syndrome: part of CATCH 22. J Med Genet. 1993 Oct;30(10):852–856. doi: 10.1136/jmg.30.10.852. [DOI] [PMC free article] [PubMed] [Google Scholar]