Abstract
Objective—To determine whether spectral analysis of unprocessed radiofrequency (RF) signal offers advantages over standard videodensitometric analysis in identifying the morphology of coronary atherosclerotic plaques. Methods—97 regions of interest (ROI) were imaged at 30 MHz from postmortem, pressure perfused (80 mm Hg) coronary arteries in saline baths. RF data were digitised at 250 MHz. Two different sizes of ROI were identified from scan converted images, and relative amplitudes of different frequency components were analysed from raw data. Normalised spectra was used to calculate spectral slope (dB/MHz), y-axis intercept (dB), mean power (dB), and maximum power (dB) over a given bandwidth (17-42 MHz). RF images were constructed and compared with comparative histology derived from microscopy and radiological techniques in three dimensions. Results—Mean power was similar from dense fibrotic tissue and heavy calcium, but spectral slope was steeper in heavy calcium (−0.45 (0.1)) than in dense fibrotic tissue (−0.31 (0.1)), and maximum power was higher for heavy calcium (−7.7 (2.0)) than for dense fibrotic tissue (−10.2 (3.9)). Maximum power was significantly higher in heavy calcium (−7.7 (2.0) dB) and dense fibrotic tissue (−10.2 (3.9) dB) than in microcalcification (−13.9 (3.8) dB). Y-axis intercept was higher in microcalcification (−5.8 (1.1) dB) than in moderately fibrotic tissue (−11.9 (2.0) dB). Moderate and dense fibrotic tissue were discriminated with mean power: moderate −20.2 (1.1) dB, dense −14.7 (3.7) dB; and y-axis intercept: moderate −11.9 (2.0) dB, dense −5.5 (5.4) dB. Different densities of fibrosis, loose, moderate, and dense, were discriminated with both y-axis intercept, spectral slope, and mean power. Lipid could be differentiated from other types of plaque tissue on the basis of spectral slope, lipid −0.17 (0.08). Also y-axis intercept from lipid (−17.6 (3.9)) differed significantly from moderately fibrotic tissue, dense fibrotic tissue, microcalcification, and heavy calcium. No significant differences in any of the measured parameters were seen between the results obtained from small and large ROIs. Conclusion—Frequency based spectral analysis of unprocessed ultrasound signal may lead to accurate identification of atherosclerotic plaque morphology. Keywords: tissue characterisation; intravascular ultrasound; spectral analysis; radiofrequency data
Full Text
The Full Text of this article is available as a PDF (729.9 KB).
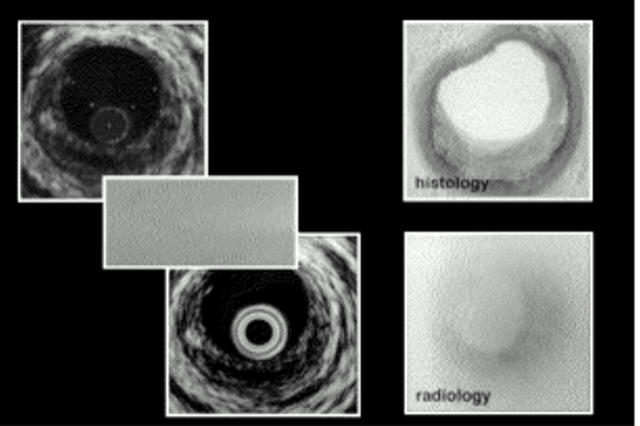
Figure 1 .
Scan converted RF image (upper left), raw ultrasound data (middle left), normal videoimage (lower left), histology, and radiology from corresponding vessel site (right).
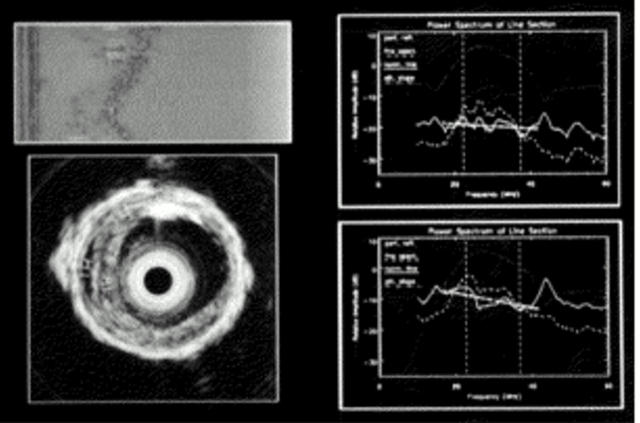
Figure 2 .
Spectral analysis. (Upper left) Raw, unprocessed radiofrequency data. (Lower left) Scan converted image of the area of interest. (Right) Power spectrum from two regions of interest.
Figure 3 .
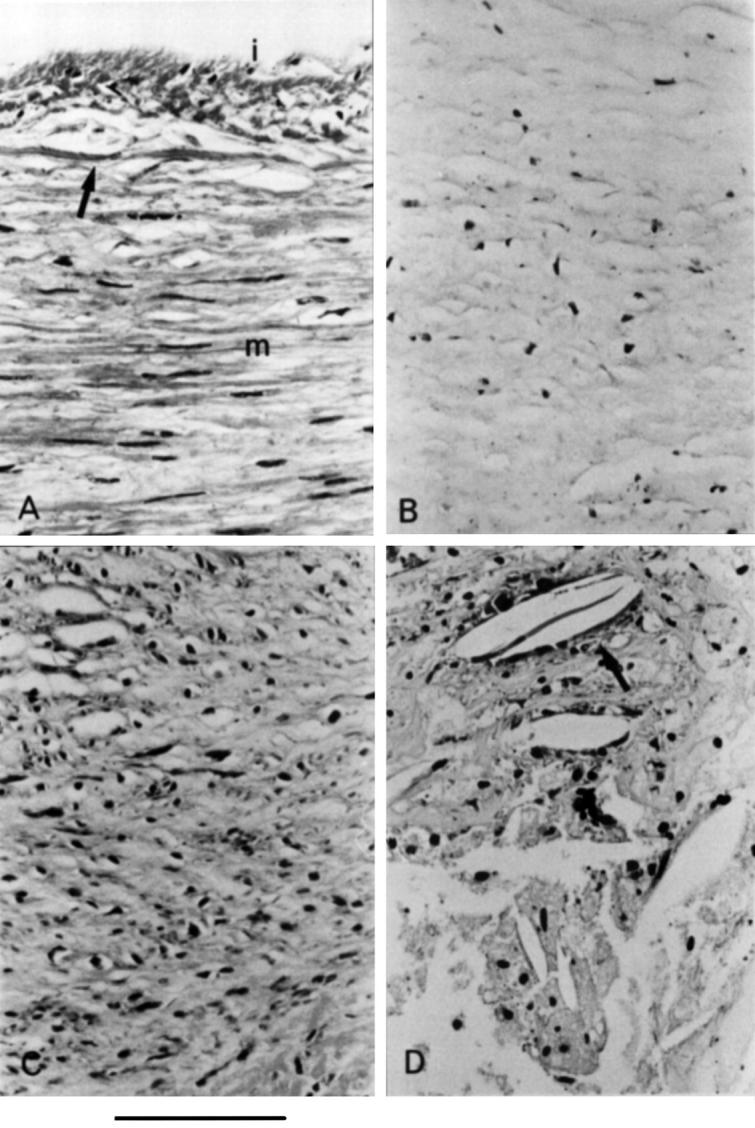
Morphometric features of sonolucent plaques. (A) Normal media, smooth muscle cells of the normal media (m) are separated from a thin intima (i) by an internal elastic lamina (arrow). (B) Loose fibrotic tissue, atheromatous plaque composed of loose, collagenous fibrous tissue with small numbers of cells. (C) Moderately fibrotic tissue, atheromatous plaque showing an increase in numbers of fibroblasts and smooth muscle cells, and increased amounts of stainable collagenous connective tissue. (D) Fatty tissue, atheromatous plaque with foam cells, free lipid, and cholesterol crystals (arrow).
Figure 4 .
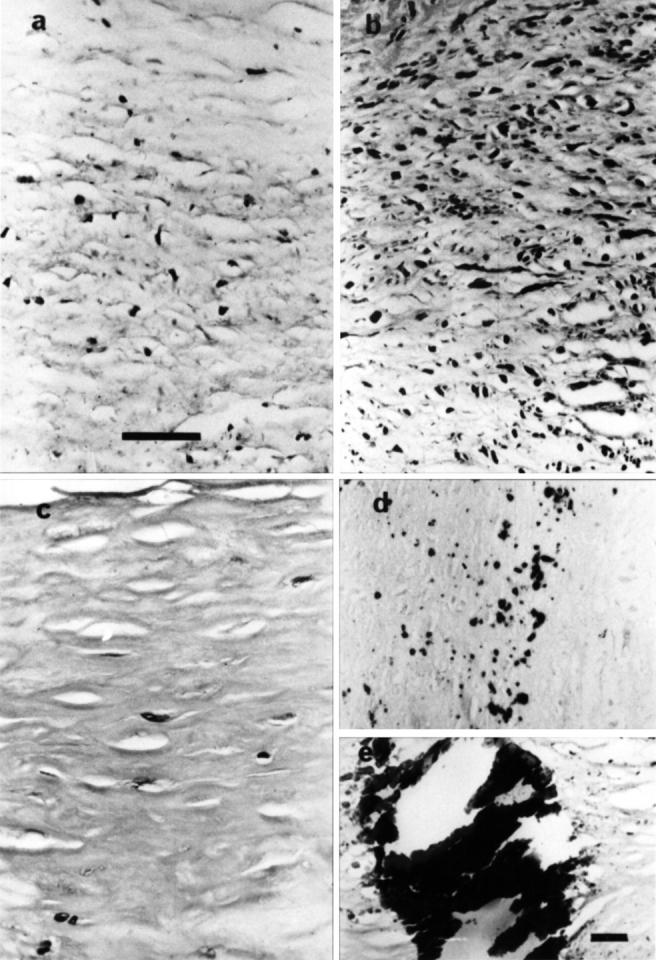
Morphological features of fibrotic and calcified plaques. (a) Loose fibrotic tissue. (b) Moderately fibrotic tissue. (c) Dense fibrotic tissue. (d) Microcalcification, areas of microcalcification with plaques visualised following staining for calcium with alizarin red. (e) Calcified plaques, heavy calcification identified with haematoxylin and eosin. (a-d) Original magnification ×50, bar = 100 µm; (e) original magnification ×12.5, bar = 200 µm.
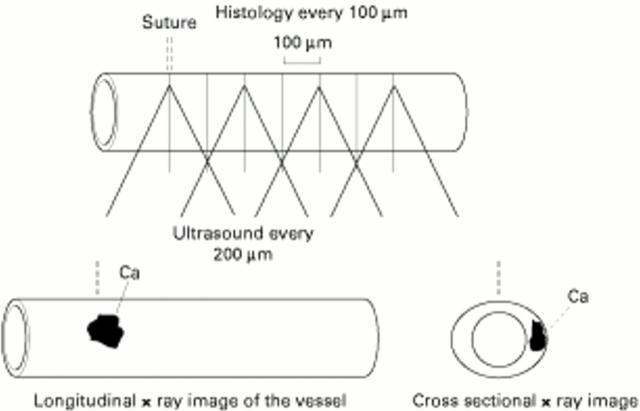
Figure 5 .
The validation of histological correspondence was achieved by comparing ultrasound images with the comparative histology derived from microscopy (histology slices were taken every 100 µm from the suture mark corresponding to the beginning of the motorised pullback of the IVUS catheter set to record data every 200 µm) and radiology techniques (radiograph of the vessel before histology to localise the clockwise and horizontal position of heavy calcium).
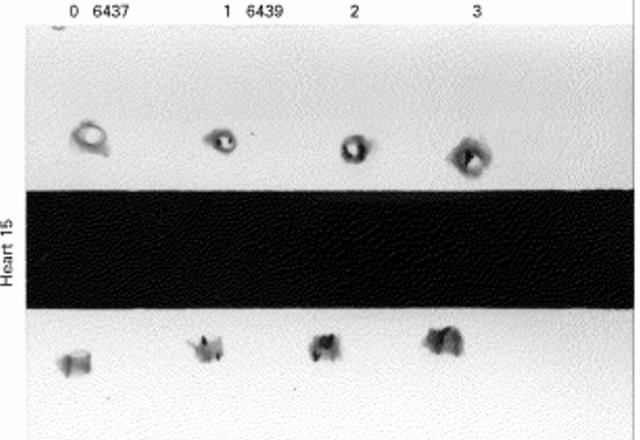
Figure 6 .
Identification and localisation of calcium with longitudinal and cross sectional radiographs of coronary arteries from heart 15: left anterior descending (6437; 0) and three sites from the right coronary artery (6439; 1, 2, 3).
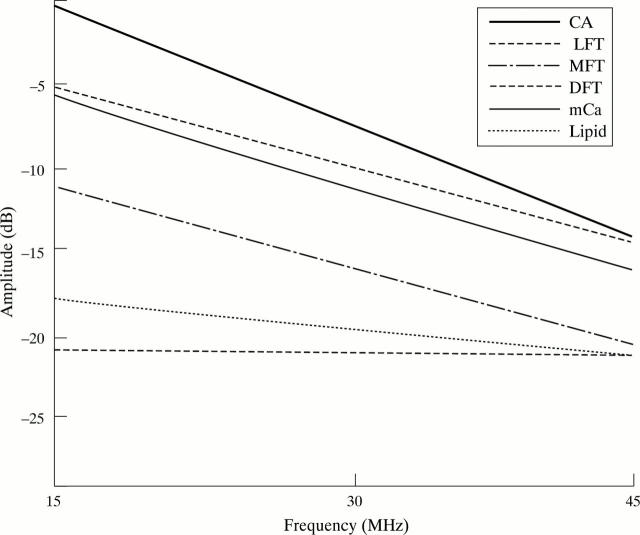
Figure 7 .
Power spectrum of lipid, loose (LFT), moderate (MFT), and dense fibrosis (DFT), heavy calcium (CA), and microcalcification (mCa).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzilai B., Saffitz J. E., Miller J. G., Sobel B. E. Quantitative ultrasonic characterization of the nature of atherosclerotic plaques in human aorta. Circ Res. 1987 Mar;60(3):459–463. doi: 10.1161/01.res.60.3.459. [DOI] [PubMed] [Google Scholar]
- Coleman D. J., Lizzi F. L. Computerized ultrasonic tissue characterization of ocular tumors. Am J Ophthalmol. 1983 Aug;96(2):165–175. doi: 10.1016/s0002-9394(14)77784-0. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Thomas A. C. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985 Apr;53(4):363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mario C., Gil R., Camenzind E., Ozaki Y., von Birgelen C., Umans V., de Jaegere P., de Feyter P. J., Roelandt J. R., Serruys P. W. Quantitative assessment with intracoronary ultrasound of the mechanisms of restenosis after percutaneous transluminal coronary angioplasty and directional coronary atherectomy. Am J Cardiol. 1995 Apr 15;75(12):772–777. doi: 10.1016/s0002-9149(99)80409-3. [DOI] [PubMed] [Google Scholar]
- Di Mario C., The S. H., Madretsma S., van Suylen R. J., Wilson R. A., Bom N., Serruys P. W., Gussenhoven E. J., Roelandt J. R. Detection and characterization of vascular lesions by intravascular ultrasound: an in vitro study correlated with histology. J Am Soc Echocardiogr. 1992 Mar-Apr;5(2):135–146. doi: 10.1016/s0894-7317(14)80543-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. J., Ports T. A., Yock P. G. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992 Jul;86(1):64–70. doi: 10.1161/01.cir.86.1.64. [DOI] [PubMed] [Google Scholar]
- Frimerman A., Miller H. I., Hallman M., Laniado S., Keren G. Intravascular ultrasound characterization of thrombi of different composition. Am J Cardiol. 1994 Jun 1;73(15):1053–1057. doi: 10.1016/0002-9149(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Goss S. A., Dunn F. Ultrasonics propagation properties of collagen. Phys Med Biol. 1980 Sep;25(5):827–837. doi: 10.1088/0031-9155/25/5/001. [DOI] [PubMed] [Google Scholar]
- Goss S. A., Johnston R. L., Dunn F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J Acoust Soc Am. 1978 Aug;64(2):423–457. doi: 10.1121/1.382016. [DOI] [PubMed] [Google Scholar]
- Gussenhoven E. J., Essed C. E., Lancée C. T., Mastik F., Frietman P., van Egmond F. C., Reiber J., Bosch H., van Urk H., Roelandt J. Arterial wall characteristics determined by intravascular ultrasound imaging: an in vitro study. J Am Coll Cardiol. 1989 Oct;14(4):947–952. doi: 10.1016/0735-1097(89)90471-3. [DOI] [PubMed] [Google Scholar]
- Gussenhoven E. J., Essed C. E., Lancée C. T., Mastik F., Frietman P., van Egmond F. C., Reiber J., Bosch H., van Urk H., Roelandt J. Arterial wall characteristics determined by intravascular ultrasound imaging: an in vitro study. J Am Coll Cardiol. 1989 Oct;14(4):947–952. doi: 10.1016/0735-1097(89)90471-3. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Vuille C., Weyman A. E. Intravascular ultrasound: basic principles and role in assessing arterial morphology and function. Am J Card Imaging. 1992 Dec;6(4):308–324. [PubMed] [Google Scholar]
- Jain S. P., Jain A., Collins T. J., Ramee S. R., White C. J. Predictors of restenosis: a morphometric and quantitative evaluation by intravascular ultrasound. Am Heart J. 1994 Oct;128(4):664–673. doi: 10.1016/0002-8703(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Kadaba M. P., Bhagat P. K., Wu V. C. Attenuation and backscattering of ultrasound in freshly excised animal tissues. IEEE Trans Biomed Eng. 1980 Feb;27(2):76–83. doi: 10.1109/TBME.1980.326710. [DOI] [PubMed] [Google Scholar]
- Landini L., Sarnelli R., Picano E., Salvadori M. Evaluation of frequency dependence of backscatter coefficient in normal and atherosclerotic aortic walls. Ultrasound Med Biol. 1986 May;12(5):397–401. doi: 10.1016/0301-5629(86)90197-3. [DOI] [PubMed] [Google Scholar]
- Linker D. T., Yock P. G., Grønningsaether A., Johansen E., Angelsen B. A. Analysis of backscattered ultrasound from normal and diseased arterial wall. Int J Card Imaging. 1989;4(2-4):177–185. doi: 10.1007/BF01745148. [DOI] [PubMed] [Google Scholar]
- Linker D. T., Yock P. G., Grønningsaether A., Johansen E., Angelsen B. A. Analysis of backscattered ultrasound from normal and diseased arterial wall. Int J Card Imaging. 1989;4(2-4):177–185. doi: 10.1007/BF01745148. [DOI] [PubMed] [Google Scholar]
- Lockwood G. R., Ryan L. K., Hunt J. W., Foster F. S. Measurement of the ultrasonic properties of vascular tissues and blood from 35-65 MHz. Ultrasound Med Biol. 1991;17(7):653–666. doi: 10.1016/0301-5629(91)90096-f. [DOI] [PubMed] [Google Scholar]
- Matar F. A., Mintz G. S., Pinnow E., Javier S. P., Popma J. J., Kent K. M., Satler L. F., Pichard A. D., Leon M. B. Multivariate predictors of intravascular ultrasound end points after directional coronary atherectomy. J Am Coll Cardiol. 1995 Feb;25(2):318–324. doi: 10.1016/0735-1097(94)00366-x. [DOI] [PubMed] [Google Scholar]
- Mintz G. S., Painter J. A., Pichard A. D., Kent K. M., Satler L. F., Popma J. J., Chuang Y. C., Bucher T. A., Sokolowicz L. E., Leon M. B. Atherosclerosis in angiographically "normal" coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. 1995 Jun;25(7):1479–1485. doi: 10.1016/0735-1097(95)00088-l. [DOI] [PubMed] [Google Scholar]
- Mintz G. S., Popma J. J., Pichard A. D., Kent K. M., Satler L. F., Chuang Y. C., Ditrano C. J., Leon M. B. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995 Apr 1;91(7):1959–1965. doi: 10.1161/01.cir.91.7.1959. [DOI] [PubMed] [Google Scholar]
- Nishimura R. A., Edwards W. D., Warnes C. A., Reeder G. S., Holmes D. R., Jr, Tajik A. J., Yock P. G. Intravascular ultrasound imaging: in vitro validation and pathologic correlation. J Am Coll Cardiol. 1990 Jul;16(1):145–154. doi: 10.1016/0735-1097(90)90472-2. [DOI] [PubMed] [Google Scholar]
- Peters R. J., Kok W. E., Bot H., Visser C. A. Characterization of plaque components with intracoronary ultrasound imaging: an in vitro quantitative study with videodensitometry. J Am Soc Echocardiogr. 1994 Nov-Dec;7(6):616–623. doi: 10.1016/s0894-7317(14)80084-9. [DOI] [PubMed] [Google Scholar]
- Rasheed Q., Dhawale P. J., Anderson J., Hodgson J. M. Intracoronary ultrasound-defined plaque composition: computer-aided plaque characterization and correlation with histologic samples obtained during directional coronary atherectomy. Am Heart J. 1995 Apr;129(4):631–637. doi: 10.1016/0002-8703(95)90307-0. [DOI] [PubMed] [Google Scholar]
- Spencer T., Ramo M. P., Salter D. M., Anderson T., Kearney P. P., Sutherland G. R., Fox K. A., McDicken W. N. Characterisation of atherosclerotic plaque by spectral analysis of intravascular ultrasound: an in vitro methodology. Ultrasound Med Biol. 1997;23(2):191–203. doi: 10.1016/s0301-5629(96)00199-8. [DOI] [PubMed] [Google Scholar]
- Tobis J. M., Mallery J., Mahon D., Lehmann K., Zalesky P., Griffith J., Gessert J., Moriuchi M., McRae M., Dwyer M. L. Intravascular ultrasound imaging of human coronary arteries in vivo. Analysis of tissue characterizations with comparison to in vitro histological specimens. Circulation. 1991 Mar;83(3):913–926. doi: 10.1161/01.cir.83.3.913. [DOI] [PubMed] [Google Scholar]
- Wilson L. S., Neale M. L., Talhami H. E., Appleberg M. Preliminary results from attenuation-slope mapping of plaque using intravascular ultrasound. Ultrasound Med Biol. 1994;20(6):529–542. doi: 10.1016/0301-5629(94)90089-2. [DOI] [PubMed] [Google Scholar]
- de Kroon M. G., van der Wal L. F., Gussenhoven W. J., Bom N. Angle-dependent backscatter from the arterial wall. Ultrasound Med Biol. 1991;17(2):121–126. doi: 10.1016/0301-5629(91)90119-h. [DOI] [PubMed] [Google Scholar]
- van der Heiden M. S., de Kroon M. G., Bom N., Borst C. Ultrasound backscatter at 30 MHz from human blood: influence of rouleau size affected by blood modification and shear rate. Ultrasound Med Biol. 1995;21(6):817–826. doi: 10.1016/0301-5629(95)00012-g. [DOI] [PubMed] [Google Scholar]