Abstract
Objective—To examine how epicardial activation and repolarisation patterns change in the course of ischaemia, and how these changes are related to the underlying histological structures. Methods—Langendorff perfused isolated rabbit hearts were submitted to 30 minutes of left anterior descending coronary artery occlusion followed by 30 minutes of reperfusion. A 256 channel epicardial map was plotted during the various experimental phases. Activation time points were determined as t(dU/dtmin) and repolarisation time points as t(dU/dtmax). From these data the local activation-recovery interval (ARI), its dispersion (SD of ARI), and the geometry of the activation spread could be analysed. After the experiments the hearts were processed histologically and the mapping data were projected onto histological slides. Results—There was elevation of the ST segment within the occluded area, which recovered during reperfusion. Within this area, ARI was significantly shortened and its dispersion was maximally enhanced. The enhancement of dispersion was pronounced at sites of histological inhomogeneity like fat, connective tissue, or vessels. There was also a change in the preferential direction of activation spread within the occluded zone with a marked transverse propagation of the activation wavefront, whereas under normal conditions the activation followed the longitudinal fibre axis. In addition, the total activation time in the occluded area was significantly prolonged. Conclusions—Ischaemia alters the local activation pattern with enhanced dispersion, especially at sites of histological irregularity, transverse shift of the activation waves, and a general slowing of conduction, which may explain the increased susceptibility to arrhythmia in hearts with enhanced histological irregularities—for example, an infarct or in multi-infarcted hearts, or after myocarditis. Keywords: dispersion; epicardial activation-recovery interval; ischaemia
Full Text
The Full Text of this article is available as a PDF (219.7 KB).
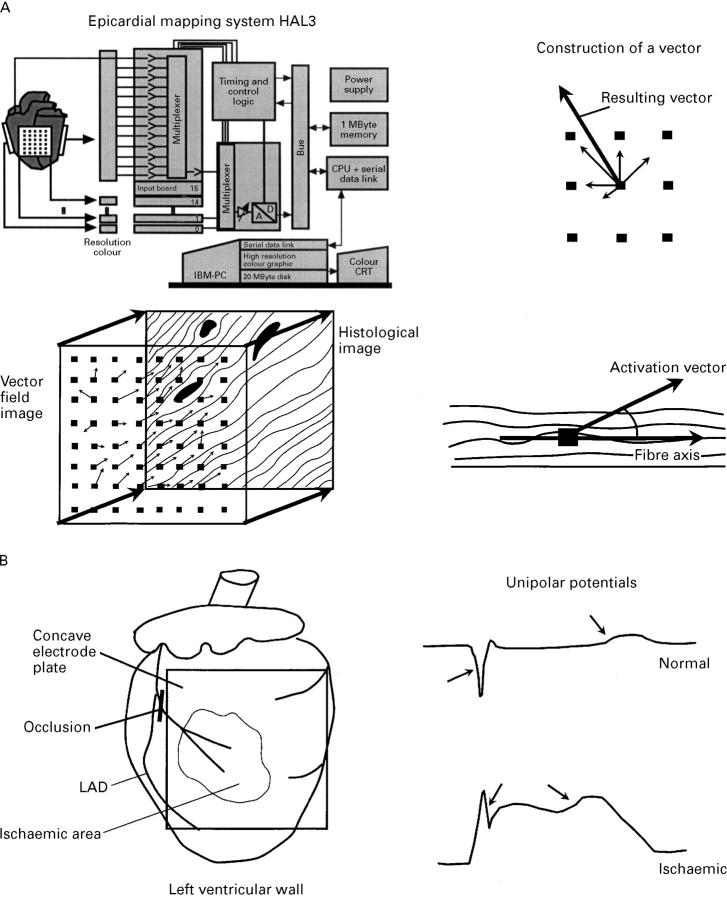
Figure 1 .
(A) Schematic representation of the experimental setup and analysis. Left upper panel: mapping system with 256 unipolar electrodes. Right upper and lower panels: the principle of vector construction: from the activation time and position of the central electrode and those electrodes, which were activated after the central electrodes, vectors were calculated and added to produce a resulting vector giving the direction and apparent velocity of the local activation. Left lower panel: projection of the resulting vector field on the composed histological image, with the construction of the angle between the fibre axis and the local activation vector. (B) Left panel: position of the concave electrode plate in relation to the ischaemic zone and the occlusion of the left anterior coronary artery branch. Right panel: typical unipolar potentials, with indication of the activation time point (determined as time point of dU/dtmin) and the repolarisation time point (determined as the time point of dU/dtmax). For details see Methods.
Figure 2 .
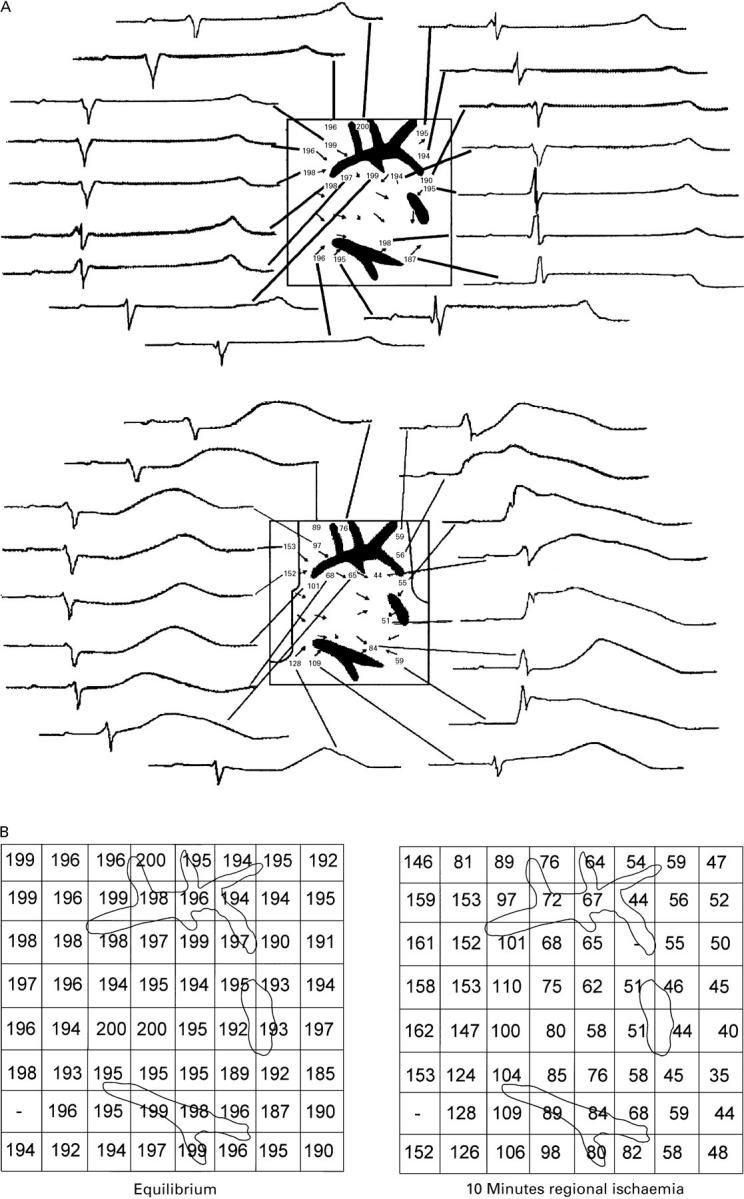
(A) Graphically reproduced images of the left ventricular wall during control (upper panel) and after 10 minutes of regional ischaemia (lower panel) in a typical experiment. The square represents the shape of the electrode grid, while black areas represent vessel sections. Numbers indicate the activation-recovery interval (ARI) in ms at that particular electrode. Vectors show local velocity and orientation of the propagating wave front at that point. Around the grid, original recordings of the unipolar ECGs of the respective electrode are displayed. The ischaemic border zone is represented as a black line in the lower part of the figure. The formerly homogeneously distributed ARI, which resemble each other closely in morphology, change to heterogeneous ARI with different morphology; the most pronounced shortening is in the direction of the propagating wavefront behind the vessel reflected by a shifted T wave in the ECG to the left and a less pronounced shortening directly in front of the vessel in the direction of the wavefront. Total activation time under control conditions was 5 ms , and after 10 minutes of ischaemia, 11 ms . (B) Left panel: ARI of the left ventricular wall under equilibrium conditions. Right panel: ARI of the left ventricular wall after 10 minutes of regional ischemia. The location of the vessels as seen in the histological image are displayed.
Figure 3 .
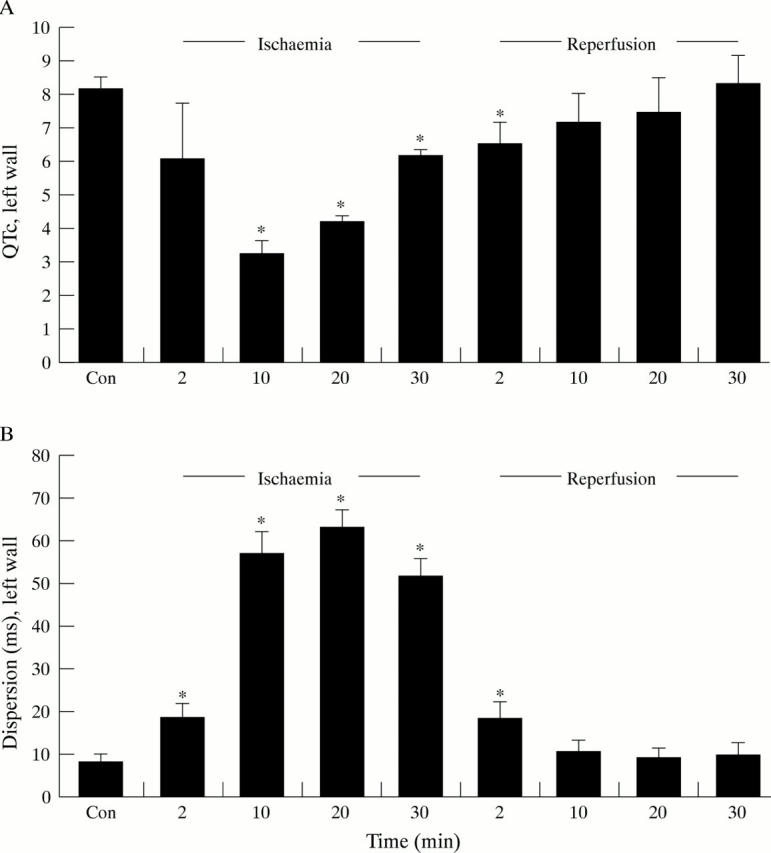
(A) QTc, calculated as ARI (activation-recovery interval)/BCL (basic cycle length), of the left ventricular wall; (B) Local dispersion of the left ventricular wall. Values are given as the means of six experiments in the course of ischaemia and reperfusion. Error bars = SEM. *p < 0.05 v values after 60 minutes of equilibration (Con).
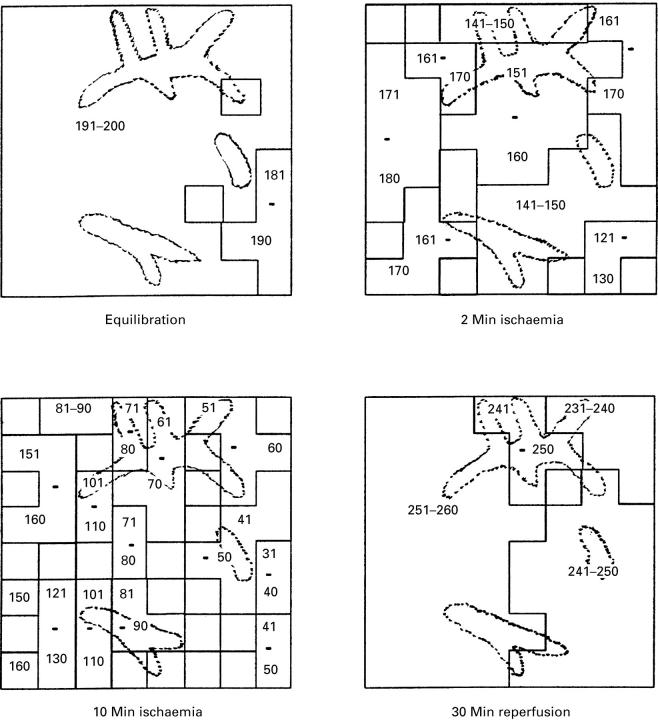
Figure 4 .
Activation-recovery interval (ARI) isochrones of a typical experiment. The isochrones indicate areas of ARI within the same range as given by the numbers. The graph shows the situation for the equilibration period, at two and 10 minutes of regional ischaemia, and after 30 minutes of reperfusion.
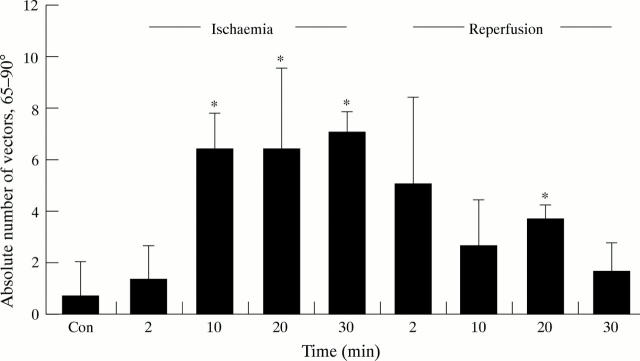
Figure 5 .
Absolute number of vectors that deviate from the fibre axis between 65° and 90° during the course of the experiment. All values are given as means of six experiments. Error bars = SEM. *p < 0.05 v control (that is, 60 minutes at equilibrium).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allessie M. A., Bonke F. I., Schopman F. J. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The "leading circle" concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977 Jul;41(1):9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- Bastide B., Hervé J. C., Délèze J. The uncoupling effect of diacylglycerol on gap junctional communication of mammalian heart cells is independent of protein kinase C. Exp Cell Res. 1994 Oct;214(2):519–527. doi: 10.1006/excr.1994.1289. [DOI] [PubMed] [Google Scholar]
- Buchanan J. W., Jr, Saito T., Gettes L. S. The effects of antiarrhythmic drugs, stimulation frequency, and potassium-induced resting membrane potential changes on conduction velocity and dV/dtmax in guinea pig myocardium. Circ Res. 1985 May;56(5):696–703. doi: 10.1161/01.res.56.5.696. [DOI] [PubMed] [Google Scholar]
- Coronel R., Fiolet J. W., Wilms-Schopman F. J., Schaapherder A. F., Johnson T. A., Gettes L. S., Janse M. J. Distribution of extracellular potassium and its relation to electrophysiologic changes during acute myocardial ischemia in the isolated perfused porcine heart. Circulation. 1988 May;77(5):1125–1138. doi: 10.1161/01.cir.77.5.1125. [DOI] [PubMed] [Google Scholar]
- DURRER D., VAN DER TWEEL L. H. Spread of activation in the left ventricular wall of the dog. II. Activation conditions at the epicardial surface. Am Heart J. 1954 Feb;47(2):192–203. doi: 10.1016/0002-8703(54)90249-5. [DOI] [PubMed] [Google Scholar]
- Delmar M., Michaels D. C., Johnson T., Jalife J. Effects of increasing intercellular resistance on transverse and longitudinal propagation in sheep epicardial muscle. Circ Res. 1987 May;60(5):780–785. doi: 10.1161/01.res.60.5.780. [DOI] [PubMed] [Google Scholar]
- Dhein S., Müller A., Gerwin R., Klaus W. Comparative study on the proarrhythmic effects of some antiarrhythmic agents. Circulation. 1993 Feb;87(2):617–630. doi: 10.1161/01.cir.87.2.617. [DOI] [PubMed] [Google Scholar]
- Dhein S., Müller A., Klaus W. Nifedipine antagonizes ouabain-induced ST-segment changes and derangement of epicardial activation pattern in isolated rabbit hearts. Int J Cardiol. 1990 Nov;29(2):163–172. doi: 10.1016/0167-5273(90)90218-t. [DOI] [PubMed] [Google Scholar]
- Dhein S., Müller A., Klaus W. Prearrhythmia: changes preceding arrhythmia, new aspects by epicardial mapping. Basic Res Cardiol. 1990 May-Jun;85(3):285–296. doi: 10.1007/BF01907117. [DOI] [PubMed] [Google Scholar]
- Dhein S., Rutten P., Klaus W. A new method for analysing the geometry and timecourse of epicardial potential spreading. Int J Biomed Comput. 1988 Dec;23(3-4):201–207. doi: 10.1016/0020-7101(88)90014-1. [DOI] [PubMed] [Google Scholar]
- HAN J., MOE G. K. NONUNIFORM RECOVERY OF EXCITABILITY IN VENTRICULAR MUSCLE. Circ Res. 1964 Jan;14:44–60. doi: 10.1161/01.res.14.1.44. [DOI] [PubMed] [Google Scholar]
- Hill J. L., Gettes L. S. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980 Apr;61(4):768–778. doi: 10.1161/01.cir.61.4.768. [DOI] [PubMed] [Google Scholar]
- Hiramatsu Y., Buchanan J. W., Knisley S. B., Gettes L. S. Rate-dependent effects of hypoxia on internal longitudinal resistance in guinea pig papillary muscles. Circ Res. 1988 Nov;63(5):923–929. doi: 10.1161/01.res.63.5.923. [DOI] [PubMed] [Google Scholar]
- Hirche H., Franz C., Bös L., Bissig R., Lang R., Schramm M. Myocardial extracellular K+ and H+ increase and noradrenaline release as possible cause of early arrhythmias following acute coronary artery occlusion in pigs. J Mol Cell Cardiol. 1980 Jun;12(6):579–593. doi: 10.1016/0022-2828(80)90016-4. [DOI] [PubMed] [Google Scholar]
- Janse M. J., van Capelle F. J., Morsink H., Kléber A. G., Wilms-Schopman F., Cardinal R., d'Alnoncourt C. N., Durrer D. Flow of "injury" current and patterns of excitation during early ventricular arrhythmias in acute regional myocardial ischemia in isolated porcine and canine hearts. Evidence for two different arrhythmogenic mechanisms. Circ Res. 1980 Aug;47(2):151–165. doi: 10.1161/01.res.47.2.151. [DOI] [PubMed] [Google Scholar]
- Kléber A. G., Janse M. J., van Capelle F. J., Durrer D. Mechanism and time course of S-T and T-Q segment changes during acute regional myocardial ischemia in the pig heart determined by extracellular and intracellular recordings. Circ Res. 1978 May;42(5):603–613. doi: 10.1161/01.res.42.5.603. [DOI] [PubMed] [Google Scholar]
- Kuo C. S., Munakata K., Reddy C. P., Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983 Jun;67(6):1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- Lesh M. D., Pring M., Spear J. F. Cellular uncoupling can unmask dispersion of action potential duration in ventricular myocardium. A computer modeling study. Circ Res. 1989 Nov;65(5):1426–1440. doi: 10.1161/01.res.65.5.1426. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Koretsune Y., Yue D. T., Chacko V. P., Pike M. M. Quantification of [Ca2+]i in perfused hearts. Critical evaluation of the 5F-BAPTA and nuclear magnetic resonance method as applied to the study of ischemia and reperfusion. Circ Res. 1990 May;66(5):1255–1267. doi: 10.1161/01.res.66.5.1255. [DOI] [PubMed] [Google Scholar]
- Marban E., Robinson S. W., Wier W. G. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986 Nov;78(5):1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey K. D., Minnich B. N., Burt J. M. Arachidonic acid and lipoxygenase metabolites uncouple neonatal rat cardiac myocyte pairs. Am J Physiol. 1992 Aug;263(2 Pt 1):C494–C501. doi: 10.1152/ajpcell.1992.263.2.C494. [DOI] [PubMed] [Google Scholar]
- Merx W., Yoon M. S., Han J. The role of local disparity in conduction and recovery time on ventricular vulnerability to fibrillation. Am Heart J. 1977 Nov;94(5):603–610. doi: 10.1016/s0002-8703(77)80130-0. [DOI] [PubMed] [Google Scholar]
- Millar C. K., Kralios F. A., Lux R. L. Correlation between refractory periods and activation-recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation. 1985 Dec;72(6):1372–1379. doi: 10.1161/01.cir.72.6.1372. [DOI] [PubMed] [Google Scholar]
- Müller A., Dhein S. Sodium channel blockade enhances dispersion of the cardiac action potential duration. A computer simulation study. Basic Res Cardiol. 1993 Jan-Feb;88(1):11–22. doi: 10.1007/BF00788526. [DOI] [PubMed] [Google Scholar]
- Saffitz J. E., Corr P. B., Sobel B. E. Arrhythmogenesis and ventricular dysfunction after myocardial infarction. Is anomalous cellular coupling the elusive link? Circulation. 1993 May;87(5):1742–1745. doi: 10.1161/01.cir.87.5.1742. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C., Heidlage J. F. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988 Apr;62(4):811–832. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C., Heidlage J. F. Interaction of inhomogeneities of repolarization with anisotropic propagation in dog atria. A mechanism for both preventing and initiating reentry. Circ Res. 1989 Dec;65(6):1612–1631. doi: 10.1161/01.res.65.6.1612. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Josephson M. E. Initiating reentry: the role of nonuniform anisotropy in small circuits. J Cardiovasc Electrophysiol. 1994 Feb;5(2):182–209. doi: 10.1111/j.1540-8167.1994.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Murphy E., Watts J. A., London R. E. Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart. Circ Res. 1990 Jan;66(1):135–146. doi: 10.1161/01.res.66.1.135. [DOI] [PubMed] [Google Scholar]
- Tan H. L., Mazón P., Verberne H. J., Sleeswijk M. E., Coronel R., Opthof T., Janse M. J. Ischaemic preconditioning delays ischaemia induced cellular electrical uncoupling in rabbit myocardium by activation of ATP sensitive potassium channels. Cardiovasc Res. 1993 Apr;27(4):644–651. doi: 10.1093/cvr/27.4.644. [DOI] [PubMed] [Google Scholar]
- Tan R. C., Joyner R. W. Electrotonic influences on action potentials from isolated ventricular cells. Circ Res. 1990 Nov;67(5):1071–1081. doi: 10.1161/01.res.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Vary T. C., Angelakos E. T., Schaffer S. W. Relationship between adenine nucleotide metabolism and irreversible ischemic tissue damage in isolated perfused rat heart. Circ Res. 1979 Aug;45(2):218–225. doi: 10.1161/01.res.45.2.218. [DOI] [PubMed] [Google Scholar]
- Wojtczak J. Contractures and increase in internal longitudianl resistance of cow ventricular muscle induced by hypoxia. Circ Res. 1979 Jan;44(1):88–95. doi: 10.1161/01.res.44.1.88. [DOI] [PubMed] [Google Scholar]
- Yamada K. A., McHowat J., Yan G. X., Donahue K., Peirick J., Kléber A. G., Corr P. B. Cellular uncoupling induced by accumulation of long-chain acylcarnitine during ischemia. Circ Res. 1994 Jan;74(1):83–95. doi: 10.1161/01.res.74.1.83. [DOI] [PubMed] [Google Scholar]
- Yan G. X., Kléber A. G. Changes in extracellular and intracellular pH in ischemic rabbit papillary muscle. Circ Res. 1992 Aug;71(2):460–470. doi: 10.1161/01.res.71.2.460. [DOI] [PubMed] [Google Scholar]