Abstract
Objective—To examine the submaximal and maximal indices of the exercise response of patients with hypertrophic cardiomyopathy. Design and setting—Prospective examination of cardiopulmonary responses to ramp exercise test of a consecutive group of patients with hypertrophic cardiomyopathy attending a cardiomyopathy outpatient clinic. Methods—50 patients aged 12 to 76 years (mean (SD) 35 (14)) with diagnosis of hypertrophic cardiomyopathy performed incremental cycle ergometry; 22 sedentary volunteers (seven female, 15 male) aged 14 to 58 years (mean (SD) 31 (12)) served as controls. Respiratory gas was continuously sampled from the mouthpiece, and its concentration profile phase aligned to the respired air flow signals. Following analogue to digital conversion, gas exchange variables were computed breath by breath and the data were averaged every 30 seconds for graphic display. A 12 lead ECG was monitored continuously and recorded every three minutes during the exercise. Results—Both the peak oxygen uptake attained on the test (V̇O2 peak) and anaerobic threshold were reduced in patients with hypertrophic cardiomyopathy compared with the control group (p < 0.0001). In 29 patients (59%) the V̇O2 peak was less than 60% and only two patients achieved a peak above 80% of their predicted values. The anaerobic threshold was below 60% of the predicted value in 31 patients and above 80% in only three patients. The slope of oxygen uptake/work rate relation (ΔV̇O2/ΔWR) was decreased in 16 patients (32%). The maximum oxygen pulse (V̇O2/HR) was reduced as a percentage of the predicted value, and became flat at high work rates in 32 patients. There was a significant correlation between anaerobic threshold and V̇O2 peak (p < 0.0001), work efficiency (p < 0.0001), and maximum oxygen pulse (p < 0.0001). The slope of change in ventilation against change in carbon dioxide output (ΔV̇E/ΔV̇CO2) for the subanaerobic threshold range was increased in 36 patients (72%) and was inversely correlated with anaerobic threshold (p < 0.0002). Conclusions—There were severe abnormalities in maximal and submaximal indices of pulmonary gas exchange in a cohort of hypertrophic cardiomyopathy patients attending a referral cardiovascular clinic. The pattern of the abnormalities suggests that a reduced stroke volume response, ventilation/perfusion mismatch, and abnormal peripheral oxygen utilisation are the possible mechanisms of exercise intolerance. Keywords: exercise tolerance; work efficiency; oxygen pulse; hypertrophic cardiomyopathy
Full Text
The Full Text of this article is available as a PDF (220.6 KB).
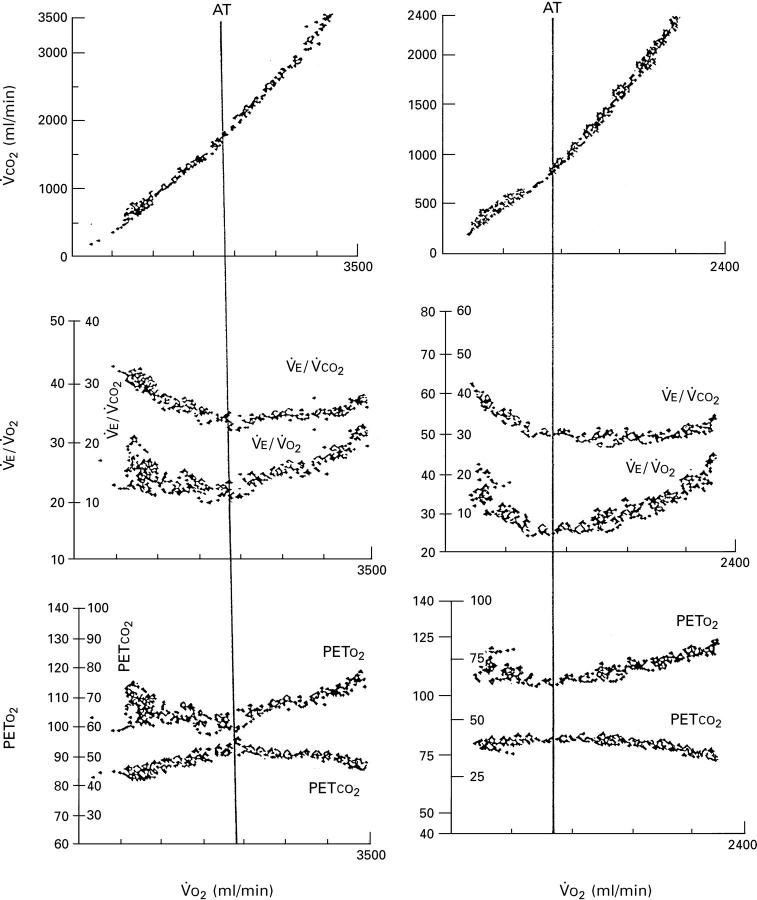
Figure 1 .
Profile of response of V̇CO2 (uppermost panels), ventilatory equivalent for V̇O2 and V̇CO2 (middle panels), and end tidal PO2 and end tidal PCO2 (lower panels) as a function of oxygen uptake during an incremental test to the limit of tolerance in a male control (left panels) and a patient with hypertrophic cardiomyopathy (right panels). Note the reduced anaerobic threshold in the patient.
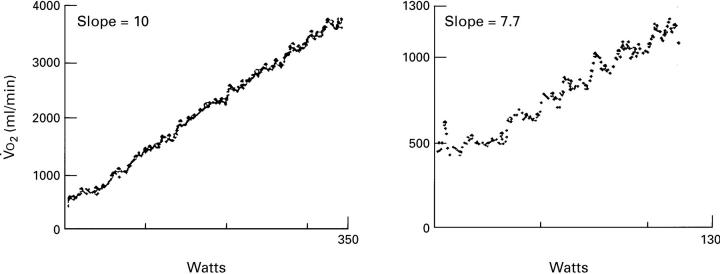
Figure 2 .
The profile of V̇O2 response, as a function of work rate, in incremental exercise test in a control subject (left panel) and a patient with hypertrophic cardiomyopathy (HCM) (right panel). Note the reduced slope of this relation in the patient with HCM.
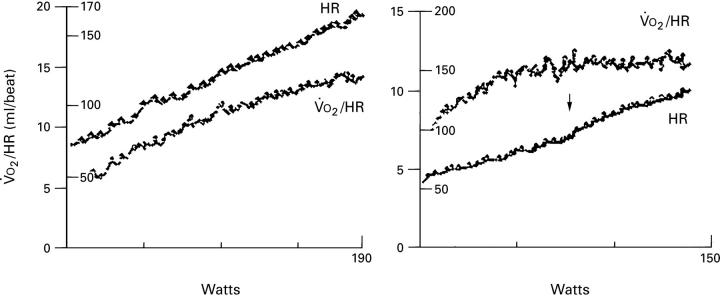
Figure 3 .
Profile of response of heart rate and oxygen pulse to progressively increasing work rate to limit of tolerance in a control subject (left panel) and in a patient with hypertrophic cardiomyopathy (HCM) (right panel). Note that the oxygen pulse continues to increase throughout the work rate in the control subject, whereas it reaches a plateau at some 60% of the maximum work rate in a patient with HCM. Note also the clearly discernible increase in the heart rate response at the work rate at which the oxygen pulse begins to plateau.
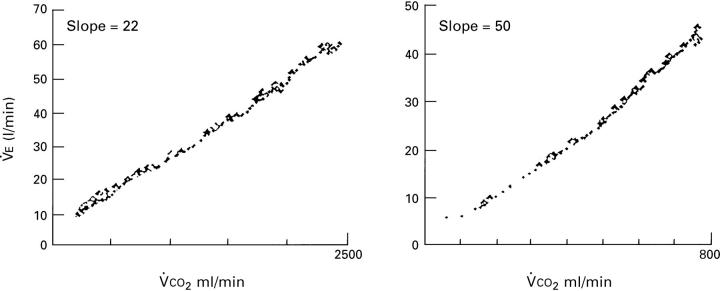
Figure 4 .
Profile of the ventilatory response as a function of V̇CO2 during an incremental exercise test to the limit of tolerance in a control subject (left panel) and a patient with hypertrophic cardiomyopathy (right panel). Note the high slope in the patient.
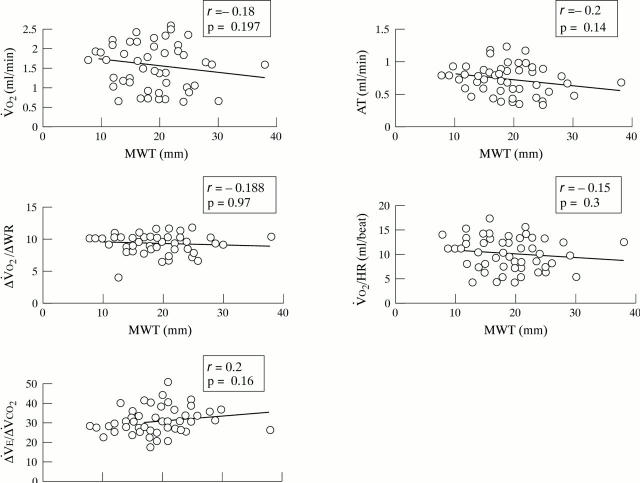
Figure 5 .
Oxygen uptake (V̇O2 ), anaerobic threshold (AT), ratio of change in oxygen uptake to work rate (ΔV̇O2/ΔWR), oxygen pulse (V̇O2 /HR), and the ratio of ventilation to carbon dioxide production (V̇E/V̇CO2 ) as a function of the left ventricular maximum wall thickness (MWT) in patients with hypertrophic cardiomyopathy.
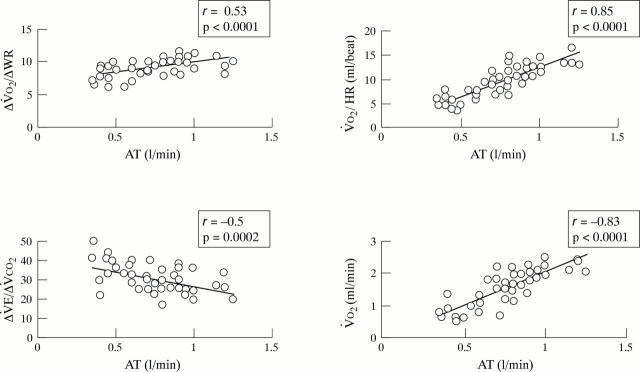
Figure 6 .
Oxygen uptake to work rate ratio (ΔV̇O2 /ΔWR), oxygen pulse (V̇O2 /HR), and oxygen uptake (V̇O2 ), and the ratio of ventilation to carbon dioxide production (V̇E/V̇CO2 ) as a function of anaerobic threshold (AT) in patients with hypertrophic cardiomyopathy.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Rawas O. A., Carter R., Richens D., Stevenson R. D., Naik S. K., Tweddel A., Wheatley D. J. Ventilatory and gas exchange abnormalities on exercise in chronic heart failure. Eur Respir J. 1995 Dec;8(12):2022–2028. doi: 10.1183/09031936.95.08122022. [DOI] [PubMed] [Google Scholar]
- Banning A. P., Lewis N. P., Northridge D. B., Elborn J. S., Hendersen A. H. Perfusion/ventilation mismatch during exercise in chronic heart failure: an investigation of circulatory determinants. Br Heart J. 1995 Jul;74(1):27–33. doi: 10.1136/hrt.74.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver W. L., Wasserman K., Whipp B. J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986 Jun;60(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Bhambhani Y. N. Prediction of stroke volume during upper and lower body exercise in men and women. Arch Phys Med Rehabil. 1995 Aug;76(8):713–718. doi: 10.1016/s0003-9993(95)80524-9. [DOI] [PubMed] [Google Scholar]
- Bhambhani Y., Norris S., Bell G. Prediction of stroke volume from oxygen pulse measurements in untrained and trained men. Can J Appl Physiol. 1994 Mar;19(1):49–59. doi: 10.1139/h94-003. [DOI] [PubMed] [Google Scholar]
- Caforio A. L., Rossi B., Risaliti R., Siciliano G., Marchetti A., Angelini C., Crea F., Mariani M., Muratorio A. Type 1 fiber abnormalities in skeletal muscle of patients with hypertrophic and dilated cardiomyopathy: evidence of subclinical myogenic myopathy. J Am Coll Cardiol. 1989 Nov 15;14(6):1464–1473. doi: 10.1016/0735-1097(89)90383-5. [DOI] [PubMed] [Google Scholar]
- Chikamori T., Dickie S., Poloniecki J. D., Myers M. J., Lavender J. P., McKenna W. J. Prognostic significance of radionuclide-assessed diastolic function in hypertrophic cardiomyopathy. Am J Cardiol. 1990 Feb 15;65(7):478–482. doi: 10.1016/0002-9149(90)90814-h. [DOI] [PubMed] [Google Scholar]
- Chua T. P., Coats A. J. The lungs in chronic heart failure. Eur Heart J. 1995 Jul;16(7):882–887. doi: 10.1093/oxfordjournals.eurheartj.a061019. [DOI] [PubMed] [Google Scholar]
- Clark A. L., Chua T. P., Coats A. J. Anatomical dead space, ventilatory pattern, and exercise capacity in chronic heart failure. Br Heart J. 1995 Oct;74(4):377–380. doi: 10.1136/hrt.74.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. L., Volterrani M., Swan J. W., Coats A. J. The increased ventilatory response to exercise in chronic heart failure: relation to pulmonary pathology. Heart. 1997 Feb;77(2):138–146. doi: 10.1136/hrt.77.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. L., Volterrani M., Swan J. W., Coats A. J. Ventilation-perfusion matching in chronic heart failure. Int J Cardiol. 1995 Mar 3;48(3):259–270. doi: 10.1016/0167-5273(94)02267-m. [DOI] [PubMed] [Google Scholar]
- Elliott P. M., Rosano G. M., Gill J. S., Poole-Wilson P. A., Kaski J. C., McKenna W. J. Changes in coronary sinus pH during dipyridamole stress in patients with hypertrophic cardiomyopathy. Heart. 1996 Feb;75(2):179–183. doi: 10.1136/hrt.75.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. E., Casaburi R., Cooper D. M., Wasserman K. Oxygen uptake as related to work rate increment during cycle ergometer exercise. Eur J Appl Physiol Occup Physiol. 1988;57(2):140–145. doi: 10.1007/BF00640653. [DOI] [PubMed] [Google Scholar]
- Lankford E. B., Epstein N. D., Fananapazir L., Sweeney H. L. Abnormal contractile properties of muscle fibers expressing beta-myosin heavy chain gene mutations in patients with hypertrophic cardiomyopathy. J Clin Invest. 1995 Mar;95(3):1409–1414. doi: 10.1172/JCI117795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele S. S., Thomson H. L., Seo H., Belenkie I., McKenna W. J., Frenneaux M. P. Exercise capacity in hypertrophic cardiomyopathy. Role of stroke volume limitation, heart rate, and diastolic filling characteristics. Circulation. 1995 Nov 15;92(10):2886–2894. doi: 10.1161/01.cir.92.10.2886. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Spirito P., Green K. J., Wesley Y. E., Bonow R. O., Arce J. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1987 Oct;10(4):733–742. doi: 10.1016/s0735-1097(87)80264-4. [DOI] [PubMed] [Google Scholar]
- Nienaber C. A., Gambhir S. S., Mody F. V., Ratib O., Huang S. C., Phelps M. E., Schelbert H. R. Regional myocardial blood flow and glucose utilization in symptomatic patients with hypertrophic cardiomyopathy. Circulation. 1993 May;87(5):1580–1590. doi: 10.1161/01.cir.87.5.1580. [DOI] [PubMed] [Google Scholar]
- Panza J. A., Maris T. J., Maron B. J. Development and determinants of dynamic obstruction to left ventricular outflow in young patients with hypertrophic cardiomyopathy. Circulation. 1992 Apr;85(4):1398–1405. doi: 10.1161/01.cir.85.4.1398. [DOI] [PubMed] [Google Scholar]
- Porszasz J., Barstow T. J., Wasserman K. Evaluation of a symmetrically disposed Pitot tube flowmeter for measuring gas flow during exercise. J Appl Physiol (1985) 1994 Dec;77(6):2659–2665. doi: 10.1152/jappl.1994.77.6.2659. [DOI] [PubMed] [Google Scholar]
- Redwood D. R., Goldstein R. E., Hirshfeld J., Borer J. S., Morganroth J., Morrow A. G., Epstein S. E. Exercise performance after septal myotomy and myectomy in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 1979 Aug;44(2):215–220. doi: 10.1016/0002-9149(79)90307-2. [DOI] [PubMed] [Google Scholar]
- Russell J. C., Dale J. D. Dynamic torquemeter calibration of bicycle ergometers. J Appl Physiol (1985) 1986 Sep;61(3):1217–1220. doi: 10.1152/jappl.1986.61.3.1217. [DOI] [PubMed] [Google Scholar]
- Spina R. J., Ogawa T., Martin W. H., 3rd, Coggan A. R., Holloszy J. O., Ehsani A. A. Exercise training prevents decline in stroke volume during exercise in young healthy subjects. J Appl Physiol (1985) 1992 Jun;72(6):2458–2462. doi: 10.1152/jappl.1992.72.6.2458. [DOI] [PubMed] [Google Scholar]
- Stratton J. R., Levy W. C., Cerqueira M. D., Schwartz R. S., Abrass I. B. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994 Apr;89(4):1648–1655. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- Sue D. Y., Wasserman K., Moricca R. B., Casaburi R. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease. Use of the V-slope method for anaerobic threshold determination. Chest. 1988 Nov;94(5):931–938. doi: 10.1378/chest.94.5.931. [DOI] [PubMed] [Google Scholar]
- Thierfelder L., Watkins H., MacRae C., Lamas R., McKenna W., Vosberg H. P., Seidman J. G., Seidman C. E. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994 Jun 3;77(5):701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Watkins H. Multiple disease genes cause hypertrophic cardiomyopathy. Br Heart J. 1994 Dec;72(6 Suppl):S4–S9. doi: 10.1136/hrt.72.6_suppl.s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipp B. J., Davis J. A., Torres F., Wasserman K. A test to determine parameters of aerobic function during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jan;50(1):217–221. doi: 10.1152/jappl.1981.50.1.217. [DOI] [PubMed] [Google Scholar]
- Whipp B. J., Davis J. A., Wasserman K. Ventilatory control of the 'isocapnic buffering' region in rapidly-incremental exercise. Respir Physiol. 1989 Jun;76(3):357–367. doi: 10.1016/0034-5687(89)90076-5. [DOI] [PubMed] [Google Scholar]
- Whipp B. J. The bioenergetic and gas exchange basis of exercise testing. Clin Chest Med. 1994 Jun;15(2):173–192. [PubMed] [Google Scholar]
- Winter U. J., Gitt A. K., Blaum M., Fritsch J., Berge P. G., Pothoff G., Hilger H. H. Kardiopulmonale Leistungsfähigkeit bei Patienten mit koronarer Herzkrankheit. Z Kardiol. 1994;83 (Suppl 3):73–82. [PubMed] [Google Scholar]