Abstract
Objective—To determine the effects of upright posture compared with supine position on the dominant atrial cycle length (DACL) in patients with chronic atrial fibrillation. Design—The power/frequency spectrum of QRST suppressed lead V1 ECG was studied in 14 patients in the supine position and during the head up tilt table test. The DACL changes were compared with changes in heart rate and blood pressure. Results—Compared with the supine position, the upright position reduced the DACL from 160 to 150 ms (p < 0.01). The DACL was increased after returning to the supine position from the upright position, from 147 to 154 ms (p < 0.01). Heart rate increased from 91 beats/min in the supine position to 106 in the upright position (p < 0.01). There was a decrease in heart rate from 109 beats/min in the upright position to 93 after returning to the supine position (p < 0.01). No significant changes were seen in systolic or diastolic blood pressure. There were indications of an inverse relation between DACL and heart rate when comparing the supine position before and after tilt with the upright position (p < 0.001). Conclusions—The sympathetic stimulation and vagal withdrawal induced by rising to upright body position are associated with a decrease in DACL during chronic atrial fibrillation. Thus a reflex increase in sympathetic discharge after induction of atrial fibrillation could favour the persistence of the arrhythmia. Keywords: atrial fibrillation; autonomic nervous system; atrial cycle length; heart rate
Full Text
The Full Text of this article is available as a PDF (142.3 KB).
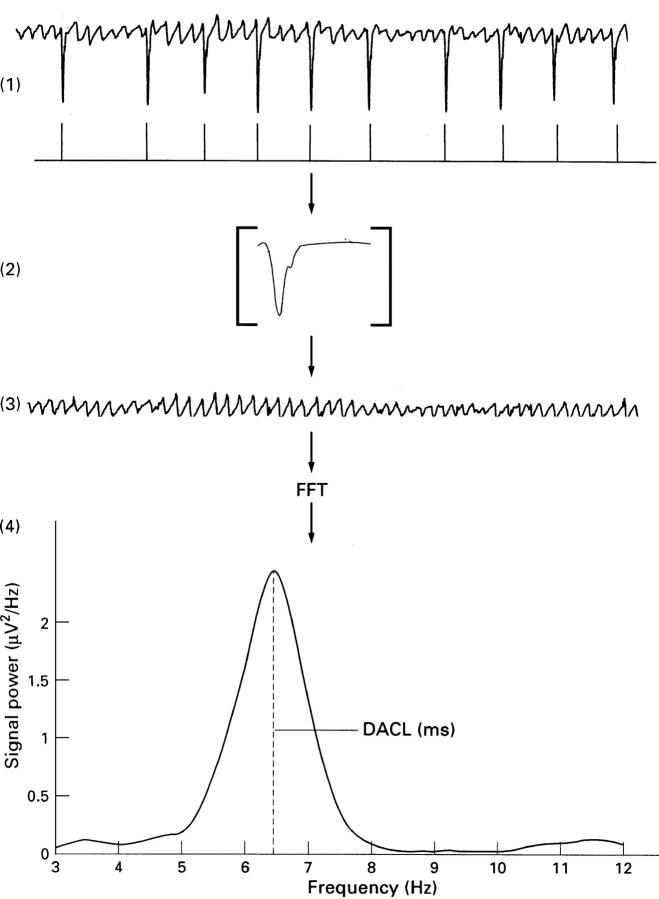
Figure 1 .
Schematic illustration of the different steps of the FAF-ECG method. (1) Low frequency components were reduced using a linear phase high pass filter. (2) Classification and subtraction of QRST complexes from the original ECG signal. (3) A frequency spectrum was estimated from the residual ECG signal using the fast Fourier transform technique (FFT). (4) The peak frequency of the dominating frequency components between 3 and 12 Hz was estimated, corresponding to the dominant atrial cycle length (DACL).
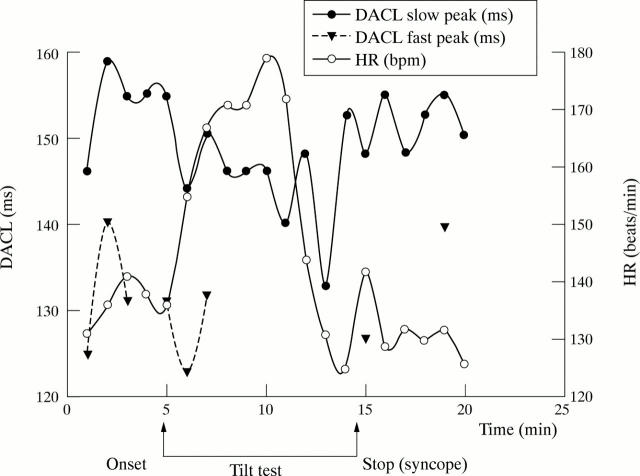
Figure 2 .
Relation of heart rate (HR) and dominant atrial cycle length (DACL) in the patient with syncope, obtained with one minute analysis intervals. The heart rate increased markedly after tilt onset and a rapid decline in both heart rate and DACL was observed before syncope, compatible with strong vagal discharge. The frequency signal had a bimodal appearance with two peaks. Note that the non-dominant peak disappears as the DACL decreases and returns after return to the supine position.
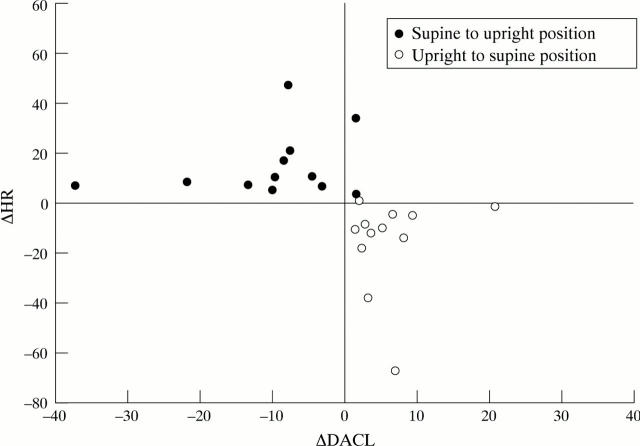
Figure 3 .
There were indications of an inverse relation between dominant atrial cycle length (DACL) and heart rate (HR) when the supine position before tilt was compared with the upright position, and when the upright position was compared with the supine position after tilt (correlation coefficient = −0.71, p < 0.001). The relation represents the different effects of increased and decreased sympathetic discharge on the atrial cycle length and AV nodal conduction time, suggesting that the effects of increased sympathetic discharge were superior to the effects of vagal withdrawal on atrial refractoriness.
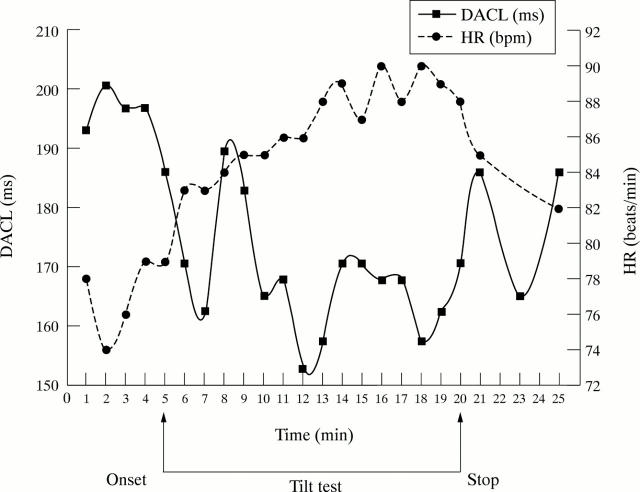
Figure 4 .
A typical patient example, illustrating one minute changes in heart rate (HR) and dominant atrial cycle length (DACL) during the tilt procedure. An increased sympathetic discharge and withdrawal of vagal activity is known to be the physiological response to the head up tilt table test. Increased sympathetic activity during chronic atrial fibrillation may favour the persistence of the arrhythmia and probably reduces the chances for a resumption of sinus rhythm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyett M. R., Kodama I., Honjo H., Arai A., Suzuki R., Toyama J. Ionic basis of the chronotropic effect of acetylcholine on the rabbit sinoatrial node. Cardiovasc Res. 1995 Jun;29(6):867–878. [PubMed] [Google Scholar]
- Capucci A., Biffi M., Boriani G., Ravelli F., Nollo G., Sabbatani P., Orsi C., Magnani B. Dynamic electrophysiological behavior of human atria during paroxysmal atrial fibrillation. Circulation. 1995 Sep 1;92(5):1193–1202. doi: 10.1161/01.cir.92.5.1193. [DOI] [PubMed] [Google Scholar]
- Charpentier F., Drouin E., Gauthier C., Le Marec H. Early after/depolarizations and triggered activity: mechanisms and autonomic regulation. Fundam Clin Pharmacol. 1993;7(1):39–49. doi: 10.1111/j.1472-8206.1993.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Cheema A. N., Ahmed M. W., Kadish A. H., Goldberger J. J. Effects of autonomic stimulation and blockade on signal-averaged P wave duration. J Am Coll Cardiol. 1995 Aug;26(2):497–502. doi: 10.1016/0735-1097(95)80028-f. [DOI] [PubMed] [Google Scholar]
- Coumel P., Attuel P., Lavallée J., Flammang D., Leclercq J. F., Slama R. Syndrome d'arythmie auriculaire d'origine vagale. Arch Mal Coeur Vaiss. 1978 Jun;71(6):645–656. [PubMed] [Google Scholar]
- Coumel P., Attuel P., Leclercq J. F., Friocourt P. Arythmies auriculaires d'origine vagale ou catécholergique. Effets comparés du traitement bêta-bloquant et phènomène d'èchappement. Arch Mal Coeur Vaiss. 1982 Apr;75(4):373–387. [PubMed] [Google Scholar]
- Coumel P. Clinical approach to paroxysmal atrial fibrillation. Clin Cardiol. 1990 Mar;13(3):209–212. doi: 10.1002/clc.4960130311. [DOI] [PubMed] [Google Scholar]
- Coumel P., Hermida J. S., Wennerblöm B., Leenhardt A., Maison-Blanche P., Cauchemez B. Heart rate variability in left ventricular hypertrophy and heart failure, and the effects of beta-blockade. A non-spectral analysis of heart rate variability in the frequency domain and in the time domain. Eur Heart J. 1991 Mar;12(3):412–422. doi: 10.1093/oxfordjournals.eurheartj.a059910. [DOI] [PubMed] [Google Scholar]
- Dhingra R. C., Amat-Y-Leon F., Wyndham C., Denes P., Wu D., Pouget J. M., Rosen K. M. Electrophysiologic effects of atropine on human sinus node and atrium. Am J Cardiol. 1976 Oct;38(4):429–434. doi: 10.1016/0002-9149(76)90458-6. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991 May 9;351(6322):145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Euler D. E., Scanlon P. J. Acetylcholine release by a stimulus train lowers atrial fibrillation threshold. Am J Physiol. 1987 Oct;253(4 Pt 2):H863–H868. doi: 10.1152/ajpheart.1987.253.4.H863. [DOI] [PubMed] [Google Scholar]
- Ewing D. J., Hume L., Campbell I. W., Murray A., Neilson J. M., Clarke B. F. Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol Respir Environ Exerc Physiol. 1980 Nov;49(5):809–814. doi: 10.1152/jappl.1980.49.5.809. [DOI] [PubMed] [Google Scholar]
- Goette A., Honeycutt C., Langberg J. J. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation. 1996 Dec 1;94(11):2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]
- Hermiller J. B., Walker S. S., Binkley P. F., Kidwell G., Schaal S. F., Wooley C. F., Stang J. M., Leier C. V. The electrophysiologic effects of upright posture. Am Heart J. 1984 Nov;108(5):1250–1254. doi: 10.1016/0002-8703(84)90749-x. [DOI] [PubMed] [Google Scholar]
- Hoffman B. F., Singer D. H. Appraisal of the effects of catecholamines on cardiac electrical activity. Ann N Y Acad Sci. 1967 Feb 10;139(3):914–939. doi: 10.1111/j.1749-6632.1967.tb41261.x. [DOI] [PubMed] [Google Scholar]
- Hordof A. J., Edie R., Malm J. R., Hoffman B. F., Rosen M. R. Electrophysiologic properties and response to pharmacologic agents of fibers from diseased human atria. Circulation. 1976 Nov;54(5):774–779. doi: 10.1161/01.cir.54.5.774. [DOI] [PubMed] [Google Scholar]
- Imaizumi S., Mazgalev T., Dreifus L. S., Michelson E. L., Miyagawa A., Bharati S., Lev M. Morphological and electrophysiological correlates of atrioventricular nodal response to increased vagal activity. Circulation. 1990 Sep;82(3):951–964. doi: 10.1161/01.cir.82.3.951. [DOI] [PubMed] [Google Scholar]
- Imanishi S., Arita M. Factors related to the low resting membrane potentials of diseased human atrial muscles. Jpn J Physiol. 1987;37(3):393–410. doi: 10.2170/jjphysiol.37.393. [DOI] [PubMed] [Google Scholar]
- Kaibara M., Nakajima T., Irisawa H., Giles W. Regulation of spontaneous opening of muscarinic K+ channels in rabbit atrium. J Physiol. 1991 Feb;433:589–613. doi: 10.1113/jphysiol.1991.sp018445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kim K. B., Rodefeld M. D., Schuessler R. B., Cox J. L., Boineau J. P. Relationship between local atrial fibrillation interval and refractory period in the isolated canine atrium. Circulation. 1996 Dec 1;94(11):2961–2967. doi: 10.1161/01.cir.94.11.2961. [DOI] [PubMed] [Google Scholar]
- Lee Y. S. Pathophysiological mechanisms of altered transmembrane potentials in diseased human atria. J Electrocardiol. 1986 Jan;19(1):41–49. doi: 10.1016/s0022-0736(86)80006-1. [DOI] [PubMed] [Google Scholar]
- Lippman N., Stein K. M., Lerman B. B. Failure to decrease parasympathetic tone during upright tilt predicts a positive tilt-table test. Am J Cardiol. 1995 Mar 15;75(8):591–595. doi: 10.1016/s0002-9149(99)80623-7. [DOI] [PubMed] [Google Scholar]
- Mary-Rabine L., Albert A., Pham T. D., Hordof A., Fenoglio J. J., Jr, Malm J. R., Rosen M. R. The relationship of human atrial cellular electrophysiology to clinical function and ultrastructure. Circ Res. 1983 Feb;52(2):188–199. doi: 10.1161/01.res.52.2.188. [DOI] [PubMed] [Google Scholar]
- McCullough J. R., Baumgarten C. M., Singer D. H. Intra- and extracellular potassium activities and the potassium equilibrium potential in partially depolarized human atrial cells. J Mol Cell Cardiol. 1987 May;19(5):477–486. doi: 10.1016/s0022-2828(87)80399-1. [DOI] [PubMed] [Google Scholar]
- Naito M., David D., Michelson E. L., Schaffenburg M., Dreifus L. S. The hemodynamic consequences of cardiac arrhythmias: evaluation of the relative roles of abnormal atrioventricular sequencing, irregularity of ventricular rhythm and atrial fibrillation in a canine model. Am Heart J. 1983 Aug;106(2):284–291. doi: 10.1016/0002-8703(83)90194-1. [DOI] [PubMed] [Google Scholar]
- Prystowsky E. N., Page R. L. Electrophysiology and autonomic influences of the human atrioventricular node. Prog Clin Biol Res. 1988;275:259–277. [PubMed] [Google Scholar]
- Rensma P. L., Allessie M. A., Lammers W. J., Bonke F. I., Schalij M. J. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988 Feb;62(2):395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- Rensma P. L., Allessie M. A., Lammers W. J., Bonke F. I., Schalij M. J. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988 Feb;62(2):395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- Rosenshtraukh L. V., Zaitsev A. V., Fast V. G., Pertsov A. M., Krinsky V. I. Vagally induced depression of impulse propagation as a cause of atrial tachycardia. J Mol Cell Cardiol. 1991 Feb;23 (Suppl 1):3–9. doi: 10.1016/0022-2828(91)90018-h. [DOI] [PubMed] [Google Scholar]
- Sako H., Imanishi S., Arita M., Shimada T., Hadama T., Uchida Y. Unaffected electrogenic Na-K pump activity in "diseased" human atrial fibers, as assessed by intracellular K+ activity. Jpn J Physiol. 1989;39(6):873–890. doi: 10.2170/jjphysiol.39.873. [DOI] [PubMed] [Google Scholar]
- Shimizu W., Tsuchioka Y., Karakawa S., Nagata K., Mukai J., Yamagata T., Matsuura H., Kajiyama G., Matsuura Y. Differential effect of pharmacological autonomic blockade on some electrophysiological properties of the human ventricle and atrium. Br Heart J. 1994 Jan;71(1):34–37. doi: 10.1136/hrt.71.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei M., Furukawa Y., Narita M., Ren L. M., Karasawa Y., Murakami M., Chiba S. Synergistic nonuniform shortening of atrial refractory period induced by autonomic stimulation. Am J Physiol. 1991 Dec;261(6 Pt 2):H1988–H1993. doi: 10.1152/ajpheart.1991.261.6.H1988. [DOI] [PubMed] [Google Scholar]
- Ten Eick R. E., Singer D. H. Electrophysiological properties of diseased human atrium. I. Low diastolic potential and altered cellular response to potassium. Circ Res. 1979 Apr;44(4):545–557. doi: 10.1161/01.res.44.4.545. [DOI] [PubMed] [Google Scholar]
- Tieleman R. G., De Langen C., Van Gelder I. C., de Kam P. J., Grandjean J., Bel K. J., Wijffels M. C., Allessie M. A., Crijns H. J. Verapamil reduces tachycardia-induced electrical remodeling of the atria. Circulation. 1997 Apr 1;95(7):1945–1953. doi: 10.1161/01.cir.95.7.1945. [DOI] [PubMed] [Google Scholar]
- Wijffels M. C., Kirchhof C. J., Dorland R., Allessie M. A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995 Oct 1;92(7):1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- Yano K., Hirata T., Hirata M., Hano O., Matsumoto Y., Mitsuoka T., Abe K., Hashiba K. Effects of sympathetic and parasympathetic stimulation on the induction of atrial flutter in dogs with aseptic pericarditis. Jpn Heart J. 1991 Nov;32(6):811–825. doi: 10.1536/ihj.32.811. [DOI] [PubMed] [Google Scholar]
- Yatani A., Okabe K., Codina J., Birnbaumer L., Brown A. M. Heart rate regulation by G proteins acting on the cardiac pacemaker channel. Science. 1990 Sep 7;249(4973):1163–1166. doi: 10.1126/science.1697697. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]