Abstract
Objective—To investigate platelet activation and deposition in human saphenous vein and internal mammary artery grafts following coronary artery bypass in vitro and in vivo, as well as inhibition of activation by the platelet selective nitric oxide donor S-nitrosoglutathione (GSNO). Design—Controlled in vitro and in vivo studies. Setting—Tertiary cardiac centre. Patients—24 patients undergoing coronary artery bypass surgery requiring vein and artery grafts. Interventions—In vitro: human platelet rich plasma was perfused through segments of vein and artery, with or without GSNO 10-6 M, and the platelet count was measured in the effluent. In vivo: indium-111 labelled antibody against the platelet α granule protein GMP-140 was injected at the end of coronary bypass grafting and γ counts were compared between vein and artery grafts with or without systemic infusion of GSNO (40 nmol/min). Results—In vitro: platelet count in perfused vein (< 70% of baseline) decreased more than in artery segments (89-94% of baseline) (p < 0.001). The platelet count was unchanged with GSNO in vein and artery segments. In vivo: γ counts were greater at all time points over vein than artery grafts (p < 0.05), and were reduced by infusion of GSNO (p < 0.05). Conclusions—Platelet activation is greater in vein than in artery grafts in vitro and in vivo. Activation, which contributes to early vein graft failure, was inhibited by GSNO. Keywords: coronary artery bypass surgery; platelet activation; S-nitrosoglutathione; ischaemic heart disease
Full Text
The Full Text of this article is available as a PDF (143.9 KB).
Figure 1 .
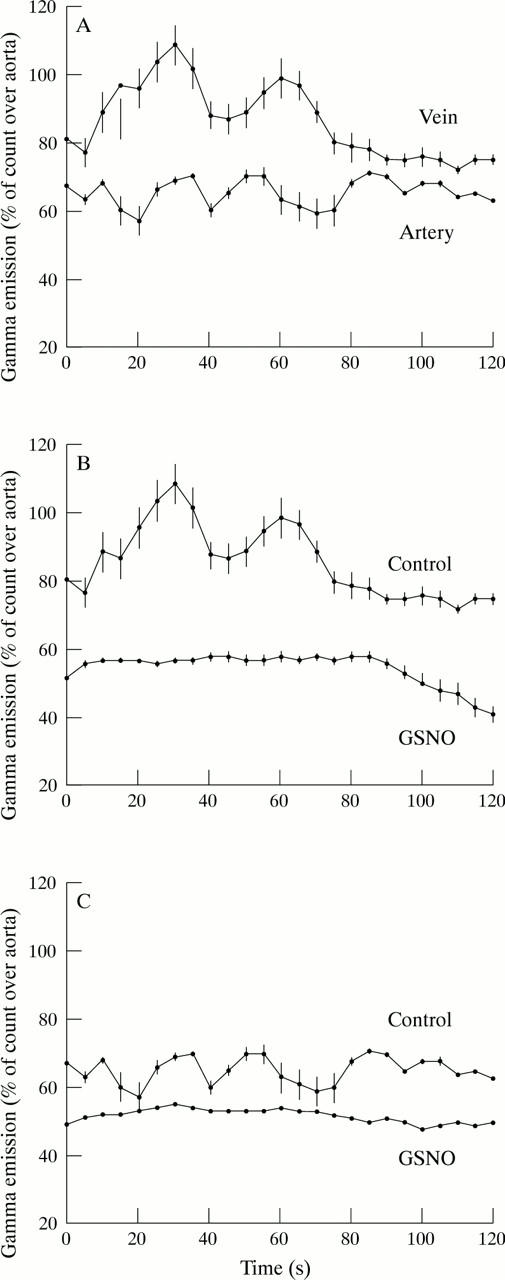
In vivo platelet GMP-140 expression detected with a γ camera in saphenous vein and internal mammary artery grafts after coronary artery bypass surgery. Counts are expressed as a percentage of counts detected in the aorta. Each point represents the mean (SEM) of six patients. (A) Platelet activation is greater in vein grafts than in artery grafts. (B) Counts over vein grafts in the presence (GSNO) or absence (control) of GSNO (40 nmol/min). (C) Counts over artery grafts in the presence (GSNO) or absence (control) of GSNO (40 nmol/min). Platelet activation in vein and arterial grafts is inhibited by GSNO.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourassa M. G. Long-term vein graft patency. Curr Opin Cardiol. 1994 Nov;9(6):685–691. doi: 10.1097/00001573-199411000-00008. [DOI] [PubMed] [Google Scholar]
- Bryan A. J., Angelini G. D. The biology of saphenous vein graft occlusion: etiology and strategies for prevention. Curr Opin Cardiol. 1994 Nov;9(6):641–649. doi: 10.1097/00001573-199411000-00002. [DOI] [PubMed] [Google Scholar]
- Chesebro J. H., Clements I. P., Fuster V., Elveback L. R., Smith H. C., Bardsley W. T., Frye R. L., Holmes D. R., Jr, Vlietstra R. E., Pluth J. R. A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N Engl J Med. 1982 Jul 8;307(2):73–78. doi: 10.1056/NEJM198207083070201. [DOI] [PubMed] [Google Scholar]
- Goldman S., Copeland J., Moritz T., Henderson W., Zadina K., Ovitt T., Doherty J., Read R., Chesler E., Sako Y. Saphenous vein graft patency 1 year after coronary artery bypass surgery and effects of antiplatelet therapy. Results of a Veterans Administration Cooperative Study. Circulation. 1989 Nov;80(5):1190–1197. doi: 10.1161/01.cir.80.5.1190. [DOI] [PubMed] [Google Scholar]
- Grondin C. M., Campeau L., Lespérance J., Enjalbert M., Bourassa M. G. Comparison of late changes in internal mammary artery and saphenous vein grafts in two consecutive series of patients 10 years after operation. Circulation. 1984 Sep;70(3 Pt 2):I208–I212. [PubMed] [Google Scholar]
- Langford E. J., Brown A. S., Wainwright R. J., de Belder A. J., Thomas M. R., Smith R. E., Radomski M. W., Martin J. F., Moncada S. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994 Nov 26;344(8935):1458–1460. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- Langford E. J., Wainwright R. J., Martin J. F. Platelet activation in acute myocardial infarction and unstable angina is inhibited by nitric oxide donors. Arterioscler Thromb Vasc Biol. 1996 Jan;16(1):51–55. doi: 10.1161/01.atv.16.1.51. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Diederich D., Siebenmann R., Lehmann K., Stulz P., von Segesser L., Yang Z. H., Turina M., Grädel E., Weber E. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med. 1988 Aug 25;319(8):462–467. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]
- Mehta J., Mehta P. Comparative effects of nitroprusside and nitroglycerin on platelet aggregation in patients with heart failure. J Cardiovasc Pharmacol. 1980 Jan-Feb;2(1):25–33. doi: 10.1097/00005344-198001000-00004. [DOI] [PubMed] [Google Scholar]
- Miller D. D., Rivera F. J., Garcia O. J., Palmaz J. C., Berger H. J., Weisman H. F. Imaging of vascular injury with 99mTc-labeled monoclonal antiplatelet antibody S12. Preliminary experience in human percutaneous transluminal angioplasty. Circulation. 1992 Apr;85(4):1354–1363. doi: 10.1161/01.cir.85.4.1354. [DOI] [PubMed] [Google Scholar]
- Mills S. L., Denardo S. J., Denardo G. L., Epstein A. L., Peng J. S., Colcher D. 123I radiolabeling of monoclonal antibodies for in vivo procedures. Hybridoma. 1986 Winter;5(4):265–275. doi: 10.1089/hyb.1986.5.265. [DOI] [PubMed] [Google Scholar]
- Palabrica T. M., Furie B. C., Konstam M. A., Aronovitz M. J., Connolly R., Brockway B. A., Ramberg K. L., Furie B. Thrombus imaging in a primate model with antibodies specific for an external membrane protein of activated platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1036–1040. doi: 10.1073/pnas.86.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Rees D. D., Dutra A., Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992 Nov;107(3):745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay B., Radomski M., De Belder A., Martin J. F., Lopez-Jaramillo P. Systemic effects of S-nitroso-glutathione in the human following intravenous infusion. Br J Clin Pharmacol. 1995 Jul;40(1):101–102. doi: 10.1111/j.1365-2125.1995.tb04545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinder C. S., Bohnert J., Rinder H. M., Mitchell J., Ault K., Hillman R. Platelet activation and aggregation during cardiopulmonary bypass. Anesthesiology. 1991 Sep;75(3):388–393. doi: 10.1097/00000542-199109000-00002. [DOI] [PubMed] [Google Scholar]
- Subramanian V. A., Hernandez Y., Tack-Goldman K., Grabowski E. F., Weksler B. B. Prostacyclin production by internal mammary artery as a factor in coronary artery bypass grafts. Surgery. 1986 Aug;100(2):376–383. [PubMed] [Google Scholar]
- Yang Z. H., Diederich D., Schneider K., Siebenmann R., Stulz P., von Segesser L., Turina M., Bühler F. R., Lüscher T. F. Endothelium-derived relaxing factor and protection against contractions induced by histamine and serotonin in the human internal mammary artery and in the saphenous vein. Circulation. 1989 Oct;80(4):1041–1048. doi: 10.1161/01.cir.80.4.1041. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., Stulz P., von Segesser L., Bauer E., Turina M., Lüscher T. F. Different interactions of platelets with arterial and venous coronary bypass vessels. Lancet. 1991 Apr 20;337(8747):939–943. doi: 10.1016/0140-6736(91)91571-b. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., von Segesser L., Bauer E., Stulz P., Turina M., Lüscher T. F. Different activation of the endothelial L-arginine and cyclooxygenase pathway in the human internal mammary artery and saphenous vein. Circ Res. 1991 Jan;68(1):52–60. doi: 10.1161/01.res.68.1.52. [DOI] [PubMed] [Google Scholar]
- de Belder A. J., MacAllister R., Radomski M. W., Moncada S., Vallance P. J. Effects of S-nitroso-glutathione in the human forearm circulation: evidence for selective inhibition of platelet activation. Cardiovasc Res. 1994 May;28(5):691–694. doi: 10.1093/cvr/28.5.691. [DOI] [PubMed] [Google Scholar]


