Abstract
Objective—Flow associated dilatation (FAD%) and intimal media thickness are established markers of early atherosclerosis. This study aimed to compare the ability of the non-invasive measurements FAD% and intimal media thickness to predict coronary artery disease. Methods—FAD% and intimal media thickness were determined using high resolution ultrasound in 122 patients with clinically suspected coronary artery disease before coronary angiography. Results are given as mean (SD). Results—Patients with coronary artery disease had reduced FAD% compared with those with angiographically normal coronary vessels (3.7 (4.1) v 7.0 (3.5)%, p < 0.001), whereas intimal media thickness tended to be increased in patients with coronary artery disease (0.58 (0.35) v 0.47 (0.11)mm, p = 0.054). There was a negative correlation between FAD% and intimal media thickness (R = −0.317, p = 0.0004). Receiver operating characteristic analysis showed that FAD% ⩽ 4.5% predicted coronary artery disease with a sensitivity of 0.71 (95% confidence interval 0.61 to 0.80) and a specificity of 0.81 (0.58 to 0.95). In contrast, intimal media thickness showed a positive correlation with the extent of coronary artery disease (number of vessels with a lesion ⩾ 50%) (R = 0.324, p = 0.0003), without a clear cut off point. Conclusions—In patients with clinically suspected coronary artery disease, FAD% discriminates between the presence or absence of coronary artery disease, whereas intimal media thickness is associated more with the extent of coronary artery disease. Keywords: coronary artery disease; endothelial dysfunction; intimal media thickness; flow associated dilatation
Full Text
The Full Text of this article is available as a PDF (139.3 KB).
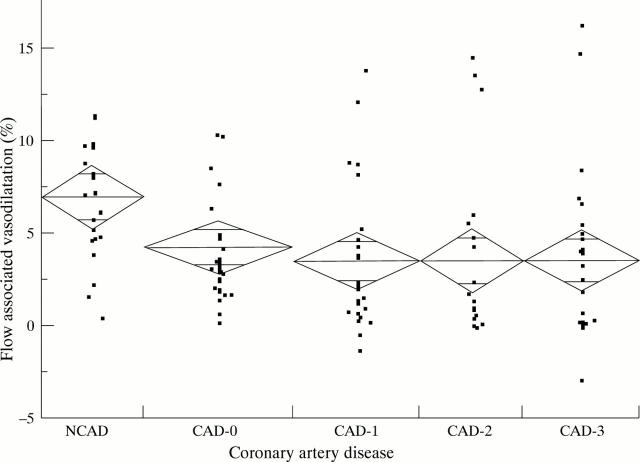
Figure 1 .
Flow associated vasodilatation (FAD%) related to the extent of coronary artery disease (CAD). NCAD, no vessel alterations; CAD-0, vessel alterations but lesion < 50%; CAD-1, lesion > 50% in one vessel; CAD-2, lesion > 50% in two vessels; CAD-3, lesion > 50% in three vessels; FAD%, flow associated (vaso)dilatation derived as percentage change during reactive hyperaemia relative to the baseline scan at rest (100%).
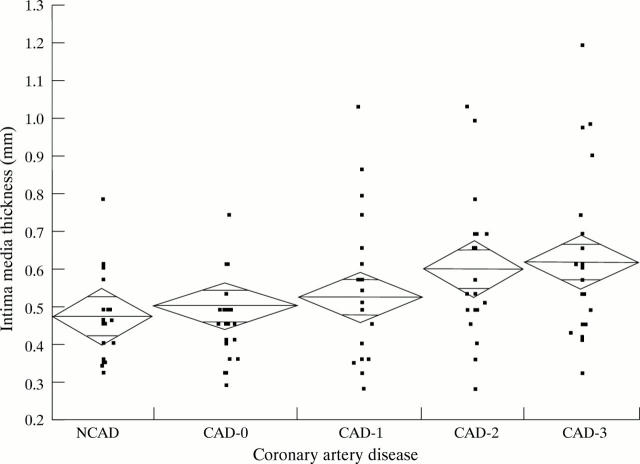
Figure 2 .
Intimal media thickness related to the extent of coronary artery disease (CAD). NCAD, no vessel alterations; CAD-0, vessel alterations, but lesion < 50%; CAD-1, lesion > 50% in one vessel; CAD-2, lesion > 50% in two vessels; CAD-3, lesion > 50% in three vessels.
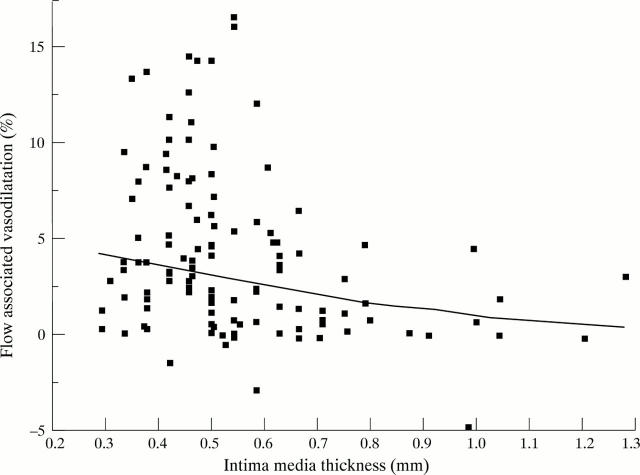
Figure 3 .
Inverse relation of flow associated vasodilatation (FAD%) and intimal media thickness (R = − 0.317, p = 0.0004, FAD% log transformed). FAD% derived as percentage change during reactive hyperaemia relative to the baseline scan at rest (100%).
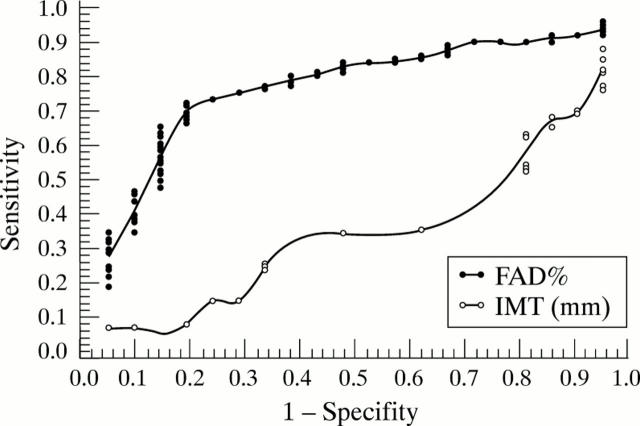
Figure 4 .
Receiver operator characteristic (ROC) analysis of flow associated vasodilatation (FAD%). Determination of a cut off point of FAD% ⩽ 4.5% as a predictor for coronary artery disease, showing a positive linear relation between intimal media thickness and coronary artery disease (R = 0.324, p = 0.0003). True positive rates (sensitivity) for the different criteria are plotted against false positive rates (1−specificity). FAD% derived as percentage change during reactive hyperaemia relative to the baseline scan at rest (100%).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. R., Nakagomi A., Keech A., Robinson J., McCredie R., Bailey B. P., Freedman S. B., Celermajer D. S. Carotid intima-media thickness is only weakly correlated with the extent and severity of coronary artery disease. Circulation. 1995 Oct 15;92(8):2127–2134. doi: 10.1161/01.cir.92.8.2127. [DOI] [PubMed] [Google Scholar]
- Anderson T. J., Uehata A., Gerhard M. D., Meredith I. T., Knab S., Delagrange D., Lieberman E. H., Ganz P., Creager M. A., Yeung A. C. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995 Nov 1;26(5):1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Bots M. L., Hofman A., Grobbee D. E. Common carotid intima-media thickness and lower extremity arterial atherosclerosis. The Rotterdam Study. Arterioscler Thromb. 1994 Dec;14(12):1885–1891. doi: 10.1161/01.atv.14.12.1885. [DOI] [PubMed] [Google Scholar]
- Calver A., Collier J., Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992 Dec;90(6):2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer D. S., Sorensen K. E., Bull C., Robinson J., Deanfield J. E. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994 Nov 15;24(6):1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Celermajer D. S., Sorensen K. E., Georgakopoulos D., Bull C., Thomas O., Robinson J., Deanfield J. E. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993 Nov;88(5 Pt 1):2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- Celermajer D. S., Sorensen K. E., Gooch V. M., Spiegelhalter D. J., Miller O. I., Sullivan I. D., Lloyd J. K., Deanfield J. E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992 Nov 7;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Clarkson P., Celermajer D. S., Donald A. E., Sampson M., Sorensen K. E., Adams M., Yue D. K., Betteridge D. J., Deanfield J. E. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. J Am Coll Cardiol. 1996 Sep;28(3):573–579. doi: 10.1016/0735-1097(96)82380-1. [DOI] [PubMed] [Google Scholar]
- Douglas P. S. Is noninvasive testing for coronary artery disease accurate? Circulation. 1997 Jan 21;95(2):299–302. doi: 10.1161/01.cir.95.2.299. [DOI] [PubMed] [Google Scholar]
- Enderle M. D., Balletshofer B. M., Schmülling R. M., Häring H. U., Pfohl M. Atherosklerose-Früherkennung mit hochauflösendem Ultraschall am Beispiel von Typ II-Diabetikern. Ultraschall Med. 1998 Feb;19(1):16–21. doi: 10.1055/s-2007-1000453. [DOI] [PubMed] [Google Scholar]
- Enderle M. D., Benda N., Schmuelling R. M., Haering H. U., Pfohl M. Preserved endothelial function in IDDM patients, but not in NIDDM patients, compared with healthy subjects. Diabetes Care. 1998 Feb;21(2):271–277. doi: 10.2337/diacare.21.2.271. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Geroulakos G., O'Gorman D., Nicolaides A., Sheridan D., Elkeles R., Shaper A. G. Carotid intima-media thickness: correlation with the British Regional Heart Study risk score. J Intern Med. 1994 May;235(5):431–433. doi: 10.1111/j.1365-2796.1994.tb01099.x. [DOI] [PubMed] [Google Scholar]
- Goldner J., Wisnant J. P., Taylor W. F. Long-term prognosis of transient cerebral ischemic attacks. Stroke. 1971 Mar-Apr;2(2):160–167. doi: 10.1161/01.str.2.2.160. [DOI] [PubMed] [Google Scholar]
- Iiyama K., Nagano M., Yo Y., Nagano N., Kamide K., Higaki J., Mikami H., Ogihara T. Impaired endothelial function with essential hypertension assessed by ultrasonography. Am Heart J. 1996 Oct;132(4):779–782. doi: 10.1016/s0002-8703(96)90311-7. [DOI] [PubMed] [Google Scholar]
- Judkins M. P. Selective coronary arteriography. I. A percutaneous transfemoral technic. Radiology. 1967 Nov;89(5):815–824. doi: 10.1148/89.5.815. [DOI] [PubMed] [Google Scholar]
- Kawamori R., Yamasaki Y., Matsushima H., Nishizawa H., Nao K., Hougaku H., Maeda H., Handa N., Matsumoto M., Kamada T. Prevalence of carotid atherosclerosis in diabetic patients. Ultrasound high-resolution B-mode imaging on carotid arteries. Diabetes Care. 1992 Oct;15(10):1290–1294. doi: 10.2337/diacare.15.10.1290. [DOI] [PubMed] [Google Scholar]
- Ludmer P. L., Selwyn A. P., Shook T. L., Wayne R. R., Mudge G. H., Alexander R. W., Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986 Oct 23;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- McNeil B. J., Keller E., Adelstein S. J. Primer on certain elements of medical decision making. N Engl J Med. 1975 Jul 31;293(5):211–215. doi: 10.1056/NEJM197507312930501. [DOI] [PubMed] [Google Scholar]
- Neunteufl T., Katzenschlager R., Hassan A., Klaar U., Schwarzacher S., Glogar D., Bauer P., Weidinger F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997 Feb 28;129(1):111–118. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- Pignoli P., Tremoli E., Poli A., Oreste P., Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986 Dec;74(6):1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993 Mar;87(3 Suppl):II56–II65. [PubMed] [Google Scholar]
- Sewell W. H. Coronary arteriography by the Sones technique. Technical considerations. Am J Roentgenol Radium Ther Nucl Med. 1965 Nov;95(3):673–683. doi: 10.2214/ajr.95.3.673. [DOI] [PubMed] [Google Scholar]
- Smits P., Kapma J. A., Jacobs M. C., Lutterman J., Thien T. Endothelium-dependent vascular relaxation in patients with type I diabetes. Diabetes. 1993 Jan;42(1):148–153. doi: 10.2337/diab.42.1.148. [DOI] [PubMed] [Google Scholar]
- Sorensen K. E., Celermajer D. S., Georgakopoulos D., Hatcher G., Betteridge D. J., Deanfield J. E. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest. 1994 Jan;93(1):50–55. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen K. E., Celermajer D. S., Spiegelhalter D. J., Georgakopoulos D., Robinson J., Thomas O., Deanfield J. E. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J. 1995 Sep;74(3):247–253. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visonà A., Pesavento R., Lusiani L., Bonanome A., Cernetti C., Rossi M., Maiolino P., Pagnan A. Intimal medial thickening of common carotid artery as indicator of coronary artery disease. Angiology. 1996 Jan;47(1):61–66. doi: 10.1177/000331979604700109. [DOI] [PubMed] [Google Scholar]
- Wendelhag I., Gustavsson T., Suurküla M., Berglund G., Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991 Nov;11(6):565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Werns S. W., Walton J. A., Hsia H. H., Nabel E. G., Sanz M. L., Pitt B. Evidence of endothelial dysfunction in angiographically normal coronary arteries of patients with coronary artery disease. Circulation. 1989 Feb;79(2):287–291. doi: 10.1161/01.cir.79.2.287. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Kawamori R., Matsushima H., Nishizawa H., Kodama M., Kajimoto Y., Morishima T., Kamada T. Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes. 1994 May;43(5):634–639. doi: 10.2337/diab.43.5.634. [DOI] [PubMed] [Google Scholar]