Abstract
Objective—To investigate the intraoperative release of troponin T during uncomplicated coronary artery surgery and to determine its relation to ischaemic time and to recovery of left ventricular function and oxidative metabolism. Design—A prospective observational study. Setting—Cardiac surgical unit in a tertiary referral centre. Methods—Troponin T, creatine kinase, and lactate were analysed from arterial and coronary sinus samples taken before operation, and 1, 4, 6, 10, 20, 35, and 45 minutes after cross clamp release. Net myocardial troponin T release and lactate extraction were derived from their respective arteriovenous differences. Haemodynamic measurements were made using a thermodilution pulmonary artery catheter. Patients—45 patients, mean (SD) age 62 (9) years, with two or three vessel coronary artery disease and chronic stable angina undergoing routine coronary artery surgery. Results—Before operation, troponin T concentrations were not raised, but within one minute of cross clamp release they increased progressively in both coronary sinus and arterial blood for the entire 45 minutes of reperfusion studied. Coronary sinus troponin T concentrations were consistently higher than arterial concentrations at all time points (p < 0.001), indicating net troponin T release by the myocardium. Peak net troponin T release and area under the curve of net troponin T release correlated closely with ischaemic time (r = 0.58 and r = 0.61, p < 0.0001 for both). Area under the curve of arterial troponin T concentration was also significantly correlated with ischaemic time (r = 0.44, p < 0.01). Patients with cross clamp times longer than 72 minutes (upper quartile for ischaemic time) had greater troponin T release, delayed reversion to lactate extraction, and lower left ventricular stroke work index three hours after surgery, compared with patients who had short (< 50 minutes, lower quartile) and intermediate (51-71 minutes, interquartile) cross clamp times. Peak net troponin T release and area under the curve of arterial troponin T concentration were inversely correlated with left ventricular stroke work index three hours after surgery (r = −0.57, r = −0.38, p < 0.01). Conclusions—Troponin T concentrations increased in every patient after cross clamp release, and were consistently higher in coronary sinus blood than in arterial blood, indicating net myocardial release of troponin T during the period of reperfusion. Intraoperative net troponin T release has functional significance, as it is closely related to ischaemic time and reflects delayed recovery of left ventricular function and oxidative metabolism; therefore, its measurement may contribute to the perioperative assessment of myocardial injury sustained during coronary artery surgery. Keywords: coronary artery surgery; troponin T; intraoperative assessment; myocardial injury
Full Text
The Full Text of this article is available as a PDF (163.1 KB).
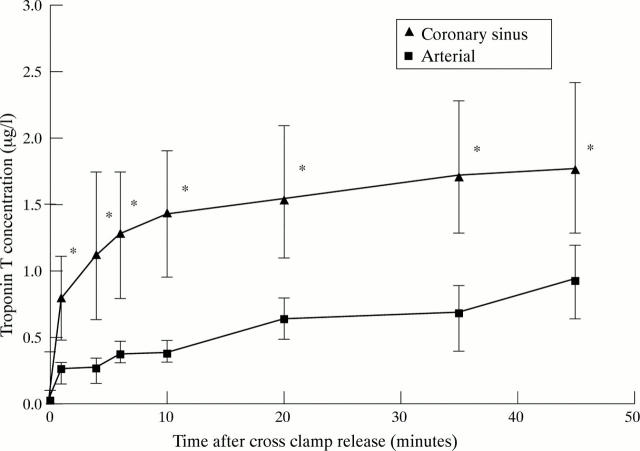
Figure 1 .
Troponin T concentration in coronary sinus and arterial blood. Troponin T concentrations in both were raised within one minute of cross clamp release and increased progressively. Coronary sinus concentrations were consistently higher than arterial: *p < 0.001 between coronary sinus and arterial concentrations. Data are expressed as median and interquartile (25th and 75th centile) range.
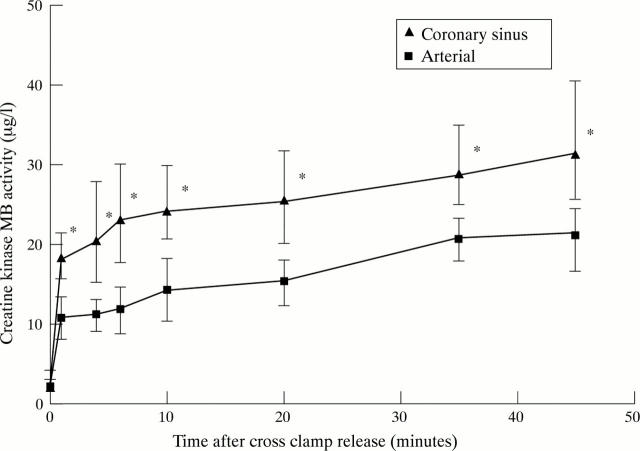
Figure 2 .
Creatine kinase MB activity in coronary sinus and arterial blood. Coronary sinus concentrations were higher than arterial at all time points: *p < 0.001 between coronary sinus and arterial concentration. Data are expressed as median and interquartile (25th and 75th centile) range.
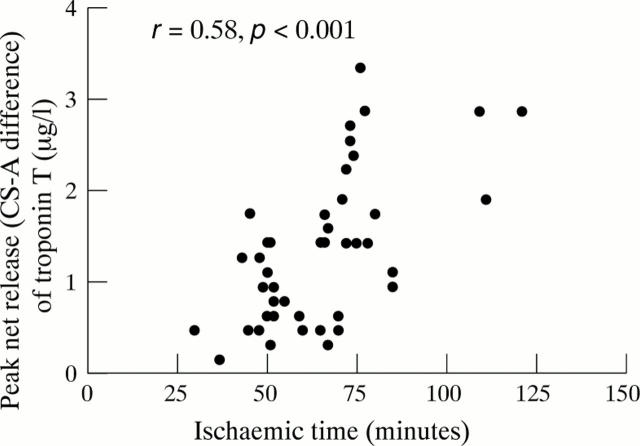
Figure 3 .
Relation between peak net release of troponin T (coronary sinus-arterial, CS-A difference) and ischaemic time, showing a close correlation.
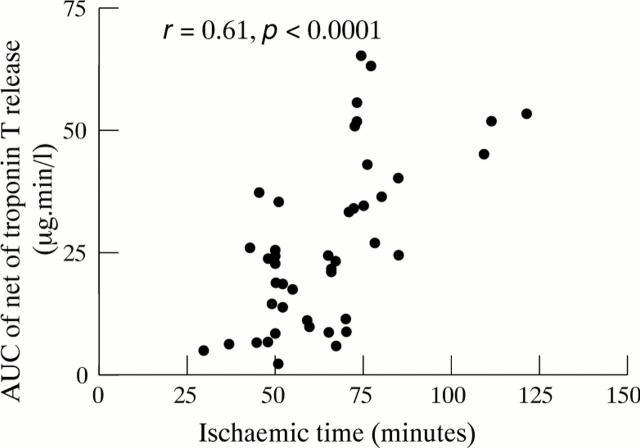
Figure 4 .
Relation between area under the curve (AUC) of net troponin T release and ischaemic time, showing that they are closely correlated.
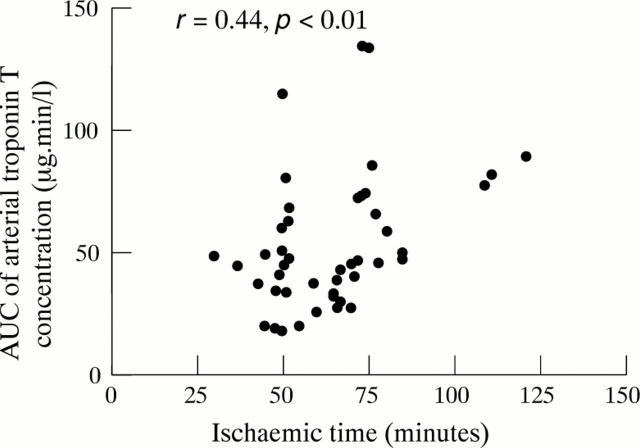
Figure 5 .
Relation between area under the curve (AUC) of arterial troponin T concentration and ischaemic time, showing a significant correlation.
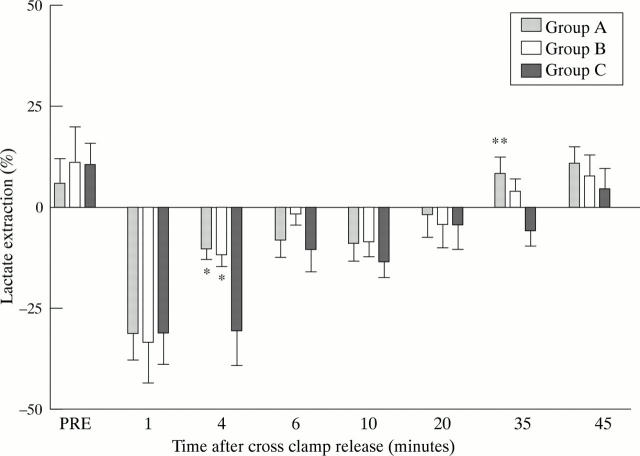
Figure 6 .
Lactate extraction in groups A, B, and C showing lower lactate production at four minutes in groups A and B v group C, and earlier reversion from production to extraction in group A at 35 minutes after cross clamp release: *p < 0.05 between group A and B v group C; **p < 0.05 between group A v group C. Data are means; error bars = SEM.
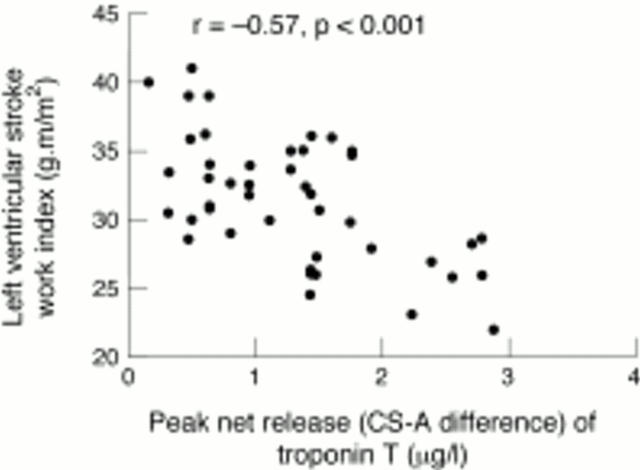
Figure 7 .
Relation between left ventricular stroke work index at three hours and peak net release of troponin T (coronary sinus-arterial, CS-A difference), showing a significant inverse correlation.
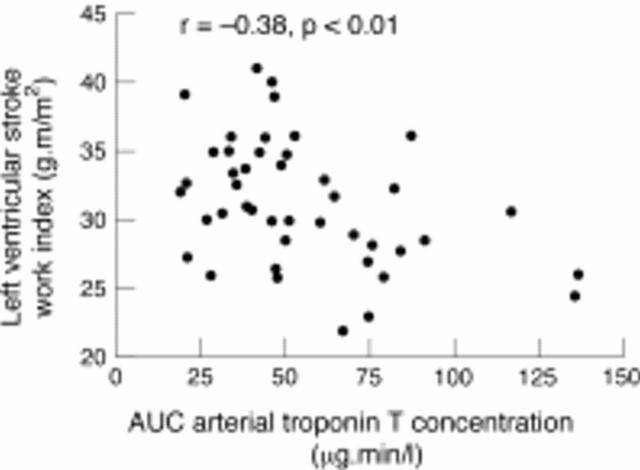
Figure 8 .
Relation between left ventricular stroke work index at three hours and area under the curve (AUC) of arterial troponin T concentration, showing significant inverse correlation.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. R., Hossein-Nia M., Kallis P., Pye M., Holt D. W., Murday A. J., Treasure T. Comparison of two strategies for myocardial management during coronary artery operations. Ann Thorac Surg. 1994 Sep;58(3):768–773. doi: 10.1016/0003-4975(94)90745-5. [DOI] [PubMed] [Google Scholar]
- Banning A., Musumeci F., Penny W., Tovey J. A. Reference intervals for cardiac troponin T, creatine kinase and creatine kinase-MB isoenzyme following coronary bypass graft surgery. Ann Clin Biochem. 1996 Nov;33(Pt 6):561–562. doi: 10.1177/000456329603300613. [DOI] [PubMed] [Google Scholar]
- Biagioli B., Borrelli E., Maccherini M., Bellomo G., Lisi G., Giomarelli P., Sani G., Toscano M. Reduction of oxidative stress does not affect recovery of myocardial function: warm continuous versus cold intermittent blood cardioplegia. Heart. 1997 May;77(5):465–473. doi: 10.1136/hrt.77.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdi I., Bryan A., Angelini G. Detection and prevention of myocardial damage during open heart surgery. Heart. 1996 Sep;76(3):189–190. doi: 10.1136/hrt.76.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt J., Rothe G., Schindler E., Döll C., Görlach G., Hempelmann G. Can clonidine, enoximone, and enalaprilat help to protect the myocardium against ischaemia in cardiac surgery? Heart. 1996 Sep;76(3):207–213. doi: 10.1136/hrt.76.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikvar L., Pillgram-Larsen J., Skjaeggestad O., Arnesen H., Strømme J. H. Serum cardio-specific troponin T after open heart surgery in patients with and without perioperative myocardial infarction. Scand J Clin Lab Invest. 1994 Jul;54(4):329–335. doi: 10.3109/00365519409087530. [DOI] [PubMed] [Google Scholar]
- Feindt P., Volkmer I., Seyfert U. T., Haack H. The role of protein C as an inhibitor of blood clotting during extracorporeal circulation. Thorac Cardiovasc Surg. 1991 Dec;39(6):338–343. doi: 10.1055/s-2007-1019995. [DOI] [PubMed] [Google Scholar]
- Ferrari R., Alfieri O., Curello S., Ceconi C., Cargnoni A., Marzollo P., Pardini A., Caradonna E., Visioli O. Occurrence of oxidative stress during reperfusion of the human heart. Circulation. 1990 Jan;81(1):201–211. doi: 10.1161/01.cir.81.1.201. [DOI] [PubMed] [Google Scholar]
- Grech E. D., Baines M., Steyn R., Faragher E. B., Page R. D., Fabri B. M., Ramsdale D. R., Rashid A. Evidence that continuous normothermic blood cardioplegia offers better myocardial protection than intermittent hypothermic cardioplegia. Br Heart J. 1995 Nov;74(5):517–521. doi: 10.1136/hrt.74.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm C. W., Ravkilde J., Gerhardt W., Jørgensen P., Peheim E., Ljungdahl L., Goldmann B., Katus H. A. The prognostic value of serum troponin T in unstable angina. N Engl J Med. 1992 Jul 16;327(3):146–150. doi: 10.1056/NEJM199207163270302. [DOI] [PubMed] [Google Scholar]
- Isomura T., Hisatomi K., Sato T., Hayashida N., Ohishi K. Interrupted warm blood cardioplegia for coronary artery bypass grafting. Eur J Cardiothorac Surg. 1995;9(3):133–138. doi: 10.1016/s1010-7940(05)80059-4. [DOI] [PubMed] [Google Scholar]
- Jenkins D. P., Pugsley W. B., Alkhulaifi A. M., Kemp M., Hooper J., Yellon D. M. Ischaemic preconditioning reduces troponin T release in patients undergoing coronary artery bypass surgery. Heart. 1997 Apr;77(4):314–318. doi: 10.1136/hrt.77.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katus H. A., Remppis A., Neumann F. J., Scheffold T., Diederich K. W., Vinar G., Noe A., Matern G., Kuebler W. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991 Mar;83(3):902–912. doi: 10.1161/01.cir.83.3.902. [DOI] [PubMed] [Google Scholar]
- Katus H. A., Remppis A., Scheffold T., Diederich K. W., Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991 Jun 15;67(16):1360–1367. doi: 10.1016/0002-9149(91)90466-x. [DOI] [PubMed] [Google Scholar]
- Katus H. A., Schoeppenthau M., Tanzeem A., Bauer H. G., Saggau W., Diederich K. W., Hagl S., Kuebler W. Non-invasive assessment of perioperative myocardial cell damage by circulating cardiac troponin T. Br Heart J. 1991 May;65(5):259–264. doi: 10.1136/hrt.65.5.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källner G., Lindblom D., Forssell G., Kallner A. Myocardial release of troponin T after coronary bypass surgery. Scand J Thorac Cardiovasc Surg. 1994;28(2):67–72. doi: 10.3109/14017439409100165. [DOI] [PubMed] [Google Scholar]
- Leung J. M., O'Kelly B., Browner W. S., Tubau J., Hollenberg M., Mangano D. T. Prognostic importance of postbypass regional wall-motion abnormalities in patients undergoing coronary artery bypass graft surgery. SPI Research Group. Anesthesiology. 1989 Jul;71(1):16–25. doi: 10.1097/00000542-198907000-00004. [DOI] [PubMed] [Google Scholar]
- Mangano D. T. Beyond CK-MB. Biochemical markers for perioperative myocardial infarction. Anesthesiology. 1994 Dec;81(6):1317–1320. [PubMed] [Google Scholar]
- Matthews J. N., Altman D. G., Campbell M. J., Royston P. Analysis of serial measurements in medical research. BMJ. 1990 Jan 27;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namay D. L., Hammermeister K. E., Zia M. S., DeRouen T. A., Dodge H. T., Namay K. Effect of perioperative myocardial infarction on late survival in patients undergoing coronary artery bypass surgery. Circulation. 1982 Jun;65(6):1066–1071. doi: 10.1161/01.cir.65.6.1066. [DOI] [PubMed] [Google Scholar]
- Noyez L., Verhagen A. F., Lacquet L. K. Antegrade versus retrograde crystalloid cardioplegia: perioperative assessment of cardiac energy metabolism by means of myocardial lactate measurement. Thorac Cardiovasc Surg. 1995 Aug;43(4):194–199. doi: 10.1055/s-2007-1013208. [DOI] [PubMed] [Google Scholar]
- Remppis A., Scheffold T., Greten J., Haass M., Greten T., Kübler W., Katus H. A. Intracellular compartmentation of troponin T: release kinetics after global ischemia and calcium paradox in the isolated perfused rat heart. J Mol Cell Cardiol. 1995 Feb;27(2):793–803. doi: 10.1016/0022-2828(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Cahalan M. K., Benefiel D. J., Byrd B. F., Lurz F. W., Shapiro W. A., Roizen M. F., Bouchard A., Schiller N. B. Intraoperative detection of myocardial ischemia in high-risk patients: electrocardiography versus two-dimensional transesophageal echocardiography. Circulation. 1985 Nov;72(5):1015–1021. doi: 10.1161/01.cir.72.5.1015. [DOI] [PubMed] [Google Scholar]
- Taggart D. P., Bhusari S., Hopper J., Kemp M., Magee P., Wright J. E., Walesby R. Intermittent ischaemic arrest and cardioplegia in coronary artery surgery: coming full circle? Br Heart J. 1994 Aug;72(2):136–139. doi: 10.1136/hrt.72.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart D. P., Young V., Hooper J., Kemp M., Walesby R., Magee P., Wright J. E. Lack of cardioprotective efficacy of allopurinol in coronary artery surgery. Br Heart J. 1994 Feb;71(2):177–181. doi: 10.1136/hrt.71.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh K. H., Christakis G. T., Weisel R. D., Fremes S. E., Mickle D. A., Romaschin A. D., Harding R. S., Ivanov J., Madonik M. M., Ross I. M. Accelerated myocardial metabolic recovery with terminal warm blood cardioplegia. J Thorac Cardiovasc Surg. 1986 Jun;91(6):888–895. [PubMed] [Google Scholar]
- Wollert H. G., Müller W., Fischer D., Wollert U., Panzner R., Schubert F., Krause E. G. Perioperative assessment of cardiac energy metabolism by means of arterio-coronary venous difference in lactate concentration (acDL). A parameter for optimizing ventricular function of the postcardioplegic myocardium. Eur J Cardiothorac Surg. 1990;4(5):278–283. doi: 10.1016/1010-7940(90)90253-v. [DOI] [PubMed] [Google Scholar]
- Yamahara Y., Asayama J., Ohta B., Matsumoto T., Miyazaki H., Tatsumi T., Kobara M., Inoue M., Inoue D., Nakagawa M. Release kinetics and correlation with hemodynamic dysfunction of cardiac troponin T in coronary effluent from isolated rat hearts during reperfusion. Basic Res Cardiol. 1993 Jul-Aug;88(4):307–313. doi: 10.1007/BF00800637. [DOI] [PubMed] [Google Scholar]
- Yau T. M., Ikonomidis J. S., Weisel R. D., Mickle D. A., Ivanov J., Mohabeer M. K., Tumiati L., Carson S., Liu P. Ventricular function after normothermic versus hypothermic cardioplegia. J Thorac Cardiovasc Surg. 1993 May;105(5):833–844. [PubMed] [Google Scholar]