Abstract
Objective—To establish the implantation technique for the atrial septal defect occluder system (ASDOS) device in an experimental animal model and to determine long term mechanical stability of the device and its in vivo properties in terms of biocompatibility and tissue reaction. Materials and methods—An atrial septal defect was created and the device implanted in 17 pigs (mean weight 30 kg). The implantation technique was refined and modified because of initial technical and anatomical complications during nine acute pilot studies. The technique proved to be feasible in eight subsequent survival studies. Four pigs were electively killed three months after implantation (group 1). The remaining four pigs were killed six months after implantation (group 2). Results—Necropsy showed all devices were embedded in soft tissue three months after implantation. Microscopic examination of atrial septal tissue showed an acute granulomatous inflammatory reaction in group 1 and fibrosis in group 2. The intensity of the inflammatory reaction around the device was clearly milder in group 2, indicating a decline in the inflammatory response with time. Clinical and biochemical investigations indicated acceptable biocompatibility of the device. Conclusion—The implantation technique for the ASDOS device in a chronic pig model has been established. Biocompatibility of the device was acceptable. Keywords: congenital heart disease; atrial septal defect; catheter technique; occluder device
Full Text
The Full Text of this article is available as a PDF (197.2 KB).
Figure 1 .
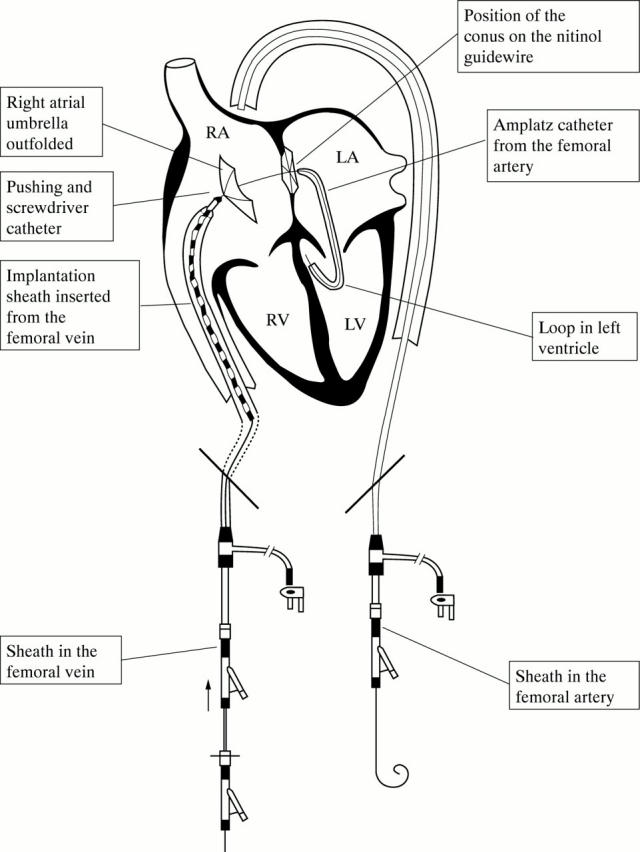
Implantation procedure. The guidewire circuit has been created and the distal (left atrial) umbrella is in place. The proximal (right atrial) umbrella is pushed towards the right side of the atrial septum. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
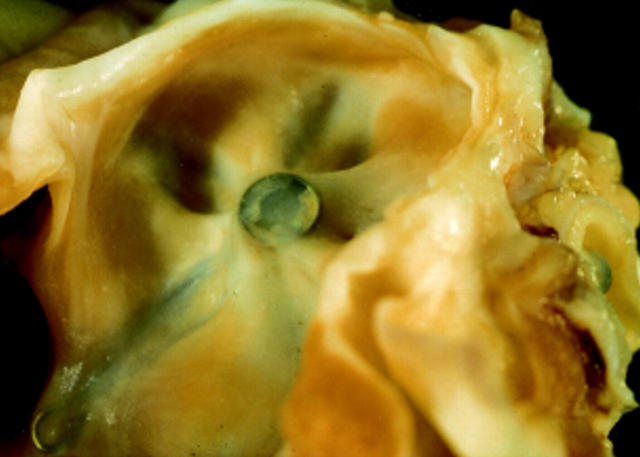
Figure 2 .
Atrial septum in a pig from group 1 (killed three months after implantation). The device is completely endothelialised and covered by smooth tissue.
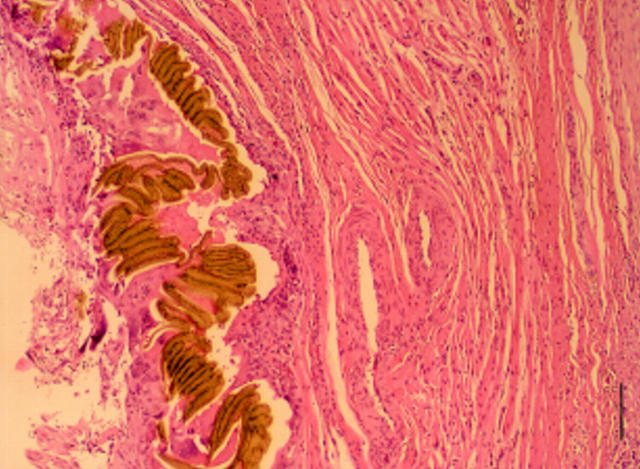
Figure 3 .
Microphotograph of atrial septal tissue in a pig in group 1 showing a fistula corresponding with the locus of a device nitinol arm. The nitinol arm is surrounded by an acute inflammatory reaction, which decreases with increasing distance from the device. (Bar = 250 µm.)
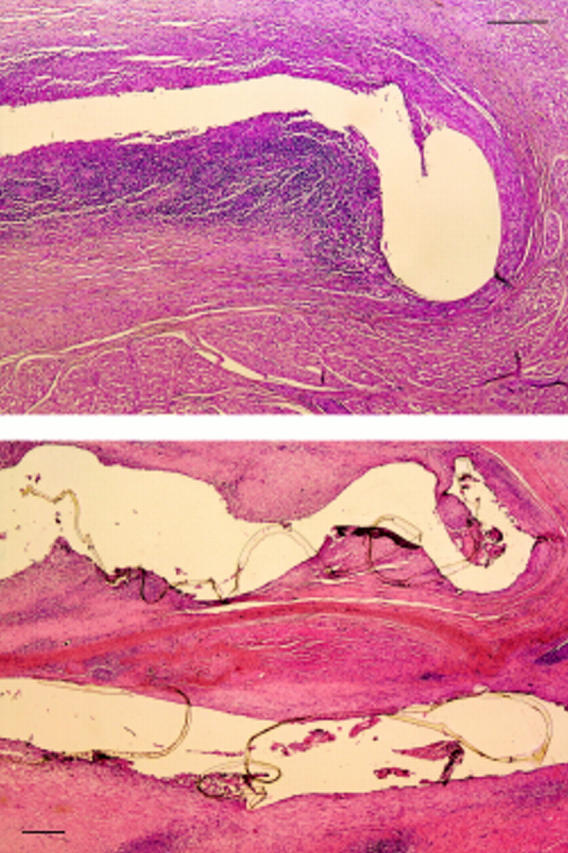
Figure 4 .

Microphotograph of the atrial septum in a pig in group 2 (killed six months after implantation) showing fistulas corresponding with the loci of two nitinol arms. Fibrosis was present and there was a milder (narrower) inflammatory reaction than in group 1. (Bar = 250 µm.)
Figure 5 .
Microphotograph of the atrial septum in a pig in group 2. A few giant cells and very mild fibrosis around the foreign body material (intact polyurethane membrane) are seen. (Bar = 100 µm.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arabia F. A., Rosado L. J., Lloyd T. R., Sethi G. K. Management of complications of Sideris transcatheter devices for atrial septal defect closure. J Thorac Cardiovasc Surg. 1993 Nov;106(5):886–888. [PubMed] [Google Scholar]
- Das G. S., Voss G., Jarvis G., Wyche K., Gunther R., Wilson R. F. Experimental atrial septal defect closure with a new, transcatheter, self-centering device. Circulation. 1993 Oct;88(4 Pt 1):1754–1764. doi: 10.1161/01.cir.88.4.1754. [DOI] [PubMed] [Google Scholar]
- Hausdorf G., Schneider M., Franzbach B., Kampmann C., Kargus K., Goeldner B. Transcatheter closure of secundum atrial septal defects with the atrial septal defect occlusion system (ASDOS): initial experience in children. Heart. 1996 Jan;75(1):83–88. doi: 10.1136/hrt.75.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. D., Thompson S. L., Steiner C., Mills N. L. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA. 1976 Jun 7;235(23):2506–2509. [PubMed] [Google Scholar]
- Kuhn M. A., Latson L. A., Cheatham J. P., McManus B., Anderson J. M., Kilzer K. L., Furst J. Biological response to Bard Clamshell Septal Occluders in the canine heart. Circulation. 1996 Apr 1;93(7):1459–1463. doi: 10.1161/01.cir.93.7.1459. [DOI] [PubMed] [Google Scholar]
- Lock J. E., Rome J. J., Davis R., Van Praagh S., Perry S. B., Van Praagh R., Keane J. F. Transcatheter closure of atrial septal defects. Experimental studies. Circulation. 1989 May;79(5):1091–1099. doi: 10.1161/01.cir.79.5.1091. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Sideris E. B., Hausdorf G., Rey C., Lloyd T. R., Beekman R. H., Worms A. M., Bourlon F., Onorato E., Khalilullah M. International experience with secundum atrial septal defect occlusion by the buttoned device. Am Heart J. 1994 Nov;128(5):1022–1035. doi: 10.1016/0002-8703(94)90602-5. [DOI] [PubMed] [Google Scholar]
- Rocchini A. P. Transcatheter closure of atrial septal defects. Past, present, and future. Circulation. 1990 Sep;82(3):1044–1045. doi: 10.1161/01.cir.82.3.1044. [DOI] [PubMed] [Google Scholar]
- Rome J. J., Keane J. F., Perry S. B., Spevak P. J., Lock J. E. Double-umbrella closure of atrial defects. Initial clinical applications. Circulation. 1990 Sep;82(3):751–758. doi: 10.1161/01.cir.82.3.751. [DOI] [PubMed] [Google Scholar]
- Roussi J., André P., Samama M., Pignaud G., Bonneau M., Laporte A., Drouet L. Platelet functions and haemostasis parameters in pigs: absence of side effects of a procedure of general anaesthesia. Thromb Res. 1996 Feb 1;81(3):297–305. doi: 10.1016/0049-3848(96)00001-1. [DOI] [PubMed] [Google Scholar]
- Sideris E. B., Sideris S. E., Fowlkes J. P., Ehly R. L., Smith J. E., Gulde R. E. Transvenous atrial septal defect occlusion in piglets with a "buttoned" double-disk device. Circulation. 1990 Jan;81(1):312–318. doi: 10.1161/01.cir.81.1.312. [DOI] [PubMed] [Google Scholar]
- Sideris E. B., Sideris S. E., Thanopoulos B. D., Ehly R. L., Fowlkes J. P. Transvenous atrial septal defect occlusion by the buttoned device. Am J Cardiol. 1990 Dec 15;66(20):1524–1526. doi: 10.1016/0002-9149(90)90552-c. [DOI] [PubMed] [Google Scholar]