Abstract
Objective—Severe impairment of left ventricular (LV) contraction is associated with an adverse prognosis in patients with ischaemic heart disease. Revascularisation may improve the impaired LV contraction if hibernating myocardium is present. The proportion of patients likely to benefit from this intervention is unknown. Therefore, the prevalence of hibernating myocardium in patients with ischaemic heart disease and severe impairment of LV contraction was assessed. Design—From a consecutive series of patients undergoing coronary angiography for the investigation of chest pain or LV impairment, all patients with ischaemic heart disease and an LV ejection fraction (LVEF) ⩽ 30% were identified. These patients underwent positron emission tomography (PET) to detect hibernating myocardium, identified by perfusion metabolism mismatch. Setting—A teaching hospital directly serving 500 000 people. Results—Of a total of 301 patients, 36 had ischaemic heart disease and an LVEF ⩽ 30%. Twenty-seven patients had PET images, while nine patients were not imaged because of emergency revascularisation (three), loss to follow up (one), inability to give consent (four), and age < 50 years (one, ethics committee guidelines). Imaged and non-imaged groups were similar in LV impairment, demographic characteristics, and risk factor profile. Fourteen patients (52% of the imaged or 39% of all patients with ischaemic heart disease and LVEF ⩽ 30%) had significant areas of hibernating myocardium on PET. Conclusion—It is possible that up to 50% of patients with ischaemic heart disease and severely impaired left ventricles have hibernating myocardium. Keywords: hibernating myocardium; left ventricular impairment; positron emission tomography
Full Text
The Full Text of this article is available as a PDF (182.9 KB).
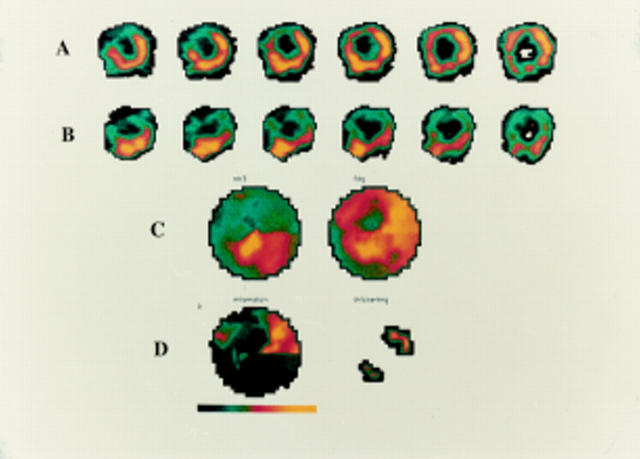
Figure 1 .
PET images of a 73 year old man with multivessel coronary artery disease and severe LV impairment. Row A represents the metabolic tomographic images after 18F-FDG injection. Row B shows the perfusion tomographic images after 13N-NH3 injection. Row C shows the perfusion polar map on the left and the metabolic polar map on the right. Row D shows the mismatch polar map on the left and the wall thickening polar map on the right. This figure represents an important area of perfusion metabolism mismatch (hibernating myocardium) in the anterolateral area.
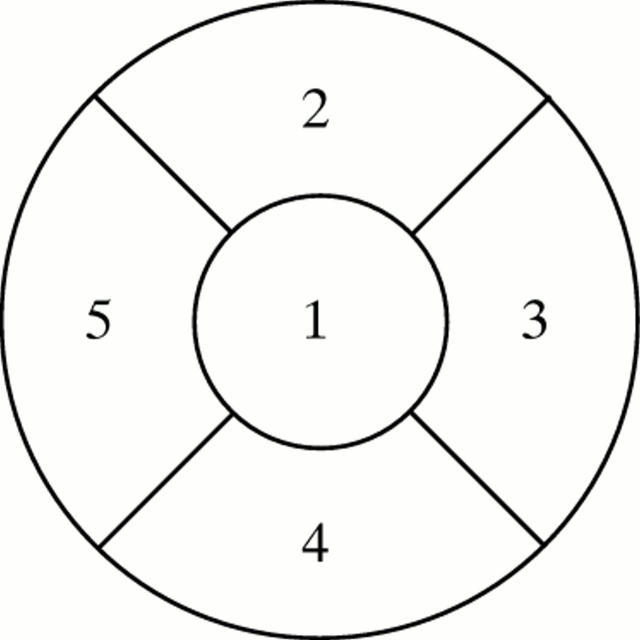
Figure 2 .
A polar map of the LV myocardium, divided into five regions: apical (1); anterior (2); lateral (3); inferior (4); and septal (5).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. N., Norton M., Trent R. J., Mikecz P., Walton S., Evans N. Incidence of hibernating myocardium after acute myocardial infarction treated with thrombolysis. Heart. 1996 May;75(5):442–446. doi: 10.1136/hrt.75.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S. R., Hack S., Tewson T., Welch M. J., Sobel B. E. The dependence of accumulation of 13NH3 by myocardium on metabolic factors and its implications for quantitative assessment of perfusion. Circulation. 1980 Jan;61(1):34–43. doi: 10.1161/01.cir.61.1.34. [DOI] [PubMed] [Google Scholar]
- Bonow R. O. Identification of viable myocardium. Circulation. 1996 Dec 1;94(11):2674–2680. doi: 10.1161/01.cir.94.11.2674. [DOI] [PubMed] [Google Scholar]
- Buvat I., Bartlett M. L., Kitsiou A. N., Dilsizian V., Bacharach S. L. A "hybrid" method for measuring myocardial wall thickening from gated PET/SPECT images. J Nucl Med. 1997 Feb;38(2):324–329. [PubMed] [Google Scholar]
- Chan R. K., Lee K. J., Calafiore P., Berlangieri S. U., McKay W. J., Tonkin A. M. Comparison of dobutamine echocardiography and positron emission tomography in patients with chronic ischemic left ventricular dysfunction. J Am Coll Cardiol. 1996 Jun;27(7):1601–1607. doi: 10.1016/0735-1097(96)00069-1. [DOI] [PubMed] [Google Scholar]
- Di Carli M. F., Asgarzadie F., Schelbert H. R., Brunken R. C., Laks H., Phelps M. E., Maddahi J. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation. 1995 Dec 15;92(12):3436–3444. doi: 10.1161/01.cir.92.12.3436. [DOI] [PubMed] [Google Scholar]
- Eitzman D., al-Aouar Z., Kanter H. L., vom Dahl J., Kirsh M., Deeb G. M., Schwaiger M. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. J Am Coll Cardiol. 1992 Sep;20(3):559–565. doi: 10.1016/0735-1097(92)90008-b. [DOI] [PubMed] [Google Scholar]
- Goris M. L., Thompson C., Malone L. J., Franken P. R. Modelling the integration of myocardial regional perfusion and function. Nucl Med Commun. 1994 Jan;15(1):9–20. doi: 10.1097/00006231-199401000-00003. [DOI] [PubMed] [Google Scholar]
- Gropler R. J., Geltman E. M., Sampathkumaran K., Pérez J. E., Moerlein S. M., Sobel B. E., Bergmann S. R., Siegel B. A. Functional recovery after coronary revascularization for chronic coronary artery disease is dependent on maintenance of oxidative metabolism. J Am Coll Cardiol. 1992 Sep;20(3):569–577. doi: 10.1016/0735-1097(92)90010-k. [DOI] [PubMed] [Google Scholar]
- Hutchins G. D., Schwaiger M., Rosenspire K. C., Krivokapich J., Schelbert H., Kuhl D. E. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990 Apr;15(5):1032–1042. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- Knuuti M. J., Nuutila P., Ruotsalainen U., Saraste M., Härkönen R., Ahonen A., Teräs M., Haaparanta M., Wegelius U., Haapanen A. Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med. 1992 Jul;33(7):1255–1262. [PubMed] [Google Scholar]
- Lemlek J., Heo J., Iskandrian A. S. The clinical relevance of myocardial viability in patient management. Am Heart J. 1992 Nov;124(5):1327–1331. doi: 10.1016/0002-8703(92)90419-v. [DOI] [PubMed] [Google Scholar]
- Maddahi J., Schelbert H., Brunken R., Di Carli M. Role of thallium-201 and PET imaging in evaluation of myocardial viability and management of patients with coronary artery disease and left ventricular dysfunction. J Nucl Med. 1994 Apr;35(4):707–715. [PubMed] [Google Scholar]
- Marwick T. H., MacIntyre W. J., Lafont A., Nemec J. J., Salcedo E. E. Metabolic responses of hibernating and infarcted myocardium to revascularization. A follow-up study of regional perfusion, function, and metabolism. Circulation. 1992 Apr;85(4):1347–1353. doi: 10.1161/01.cir.85.4.1347. [DOI] [PubMed] [Google Scholar]
- Marwick T. H., Nemec J. J., Lafont A., Salcedo E. E., MacIntyre W. J. Prediction by postexercise fluoro-18 deoxyglucose positron emission tomography of improvement in exercise capacity after revascularization. Am J Cardiol. 1992 Apr 1;69(9):854–859. doi: 10.1016/0002-9149(92)90782-t. [DOI] [PubMed] [Google Scholar]
- Murphy M. L., Hultgren H. N., Detre K., Thomsen J., Takaro T. Treatment of chronic stable angina. A preliminary report of survival data of the randomized Veterans Administration cooperative study. N Engl J Med. 1977 Sep 22;297(12):621–627. doi: 10.1056/NEJM197709222971201. [DOI] [PubMed] [Google Scholar]
- Nienaber C. A., Brunken R. C., Sherman C. T., Yeatman L. A., Gambhir S. S., Krivokapich J., Demer L. L., Ratib O., Child J. S., Phelps M. E. Metabolic and functional recovery of ischemic human myocardium after coronary angioplasty. J Am Coll Cardiol. 1991 Oct;18(4):966–978. doi: 10.1016/0735-1097(91)90755-x. [DOI] [PubMed] [Google Scholar]
- Rahimtoola S. H. The hibernating myocardium. Am Heart J. 1989 Jan;117(1):211–221. doi: 10.1016/0002-8703(89)90685-6. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation. 1991 Jul;84(1):1–14. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- Sandler H., Dodge H. T. The use of single plane angiocardiograms for the calculation of left ventricular volume in man. Am Heart J. 1968 Mar;75(3):325–334. doi: 10.1016/0002-8703(68)90089-6. [DOI] [PubMed] [Google Scholar]
- Schelbert H. R., Phelps M. E., Hoffman E. J., Huang S. C., Selin C. E., Kuhl D. E. Regional myocardial perfusion assessed with N-13 labeled ammonia and positron emission computerized axial tomography. Am J Cardiol. 1979 Feb;43(2):209–218. doi: 10.1016/s0002-9149(79)80006-5. [DOI] [PubMed] [Google Scholar]
- Schelbert H. R., Phelps M. E., Huang S. C., MacDonald N. S., Hansen H., Selin C., Kuhl D. E. N-13 ammonia as an indicator of myocardial blood flow. Circulation. 1981 Jun;63(6):1259–1272. doi: 10.1161/01.cir.63.6.1259. [DOI] [PubMed] [Google Scholar]
- Takeishi Y., Tono-oka I., Kubota I., Ikeda K., Masakane I., Chiba J., Abe S., Tsuiki K., Komatani A., Yamaguchi I. Functional recovery of hibernating myocardium after coronary bypass surgery: does it coincide with improvement in perfusion? Am Heart J. 1991 Sep;122(3 Pt 1):665–670. doi: 10.1016/0002-8703(91)90509-g. [DOI] [PubMed] [Google Scholar]
- Tamaki N., Yonekura Y., Yamashita K., Ohtani H., Hirata K., Ban T., Konishi J. Prediction of reversible ischemia after coronary artery bypass grafting by positron emission tomography. J Cardiol. 1991;21(2):193–201. [PubMed] [Google Scholar]
- Tamaki N., Yonekura Y., Yamashita K., Saji H., Magata Y., Senda M., Konishi Y., Hirata K., Ban T., Konishi J. Positron emission tomography using fluorine-18 deoxyglucose in evaluation of coronary artery bypass grafting. Am J Cardiol. 1989 Oct 15;64(14):860–865. doi: 10.1016/0002-9149(89)90832-1. [DOI] [PubMed] [Google Scholar]
- Tillisch J., Brunken R., Marshall R., Schwaiger M., Mandelkern M., Phelps M., Schelbert H. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986 Apr 3;314(14):884–888. doi: 10.1056/NEJM198604033141405. [DOI] [PubMed] [Google Scholar]
- Wolpers H. G., Burchert W., van den Hoff J., Weinhardt R., Meyer G. J., Lichtlen P. R. Assessment of myocardial viability by use of 11C-acetate and positron emission tomography. Threshold criteria of reversible dysfunction. Circulation. 1997 Mar 18;95(6):1417–1424. doi: 10.1161/01.cir.95.6.1417. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tamaki N., Yonekura Y., Ohtani H., Magata Y., Nohara R., Kambara H., Kawai C., Ban T., Konishi J. Regional wall thickening of left ventricle evaluated by gated positron emission tomography in relation to myocardial perfusion and glucose metabolism. J Nucl Med. 1991 Apr;32(4):679–685. [PubMed] [Google Scholar]
- Yoshida K., Gould K. L. Quantitative relation of myocardial infarct size and myocardial viability by positron emission tomography to left ventricular ejection fraction and 3-year mortality with and without revascularization. J Am Coll Cardiol. 1993 Oct;22(4):984–997. doi: 10.1016/0735-1097(93)90407-r. [DOI] [PubMed] [Google Scholar]
- vom Dahl J., Eitzman D. T., al-Aouar Z. R., Kanter H. L., Hicks R. J., Deeb G. M., Kirsh M. M., Schwaiger M. Relation of regional function, perfusion, and metabolism in patients with advanced coronary artery disease undergoing surgical revascularization. Circulation. 1994 Nov;90(5):2356–2366. doi: 10.1161/01.cir.90.5.2356. [DOI] [PubMed] [Google Scholar]