Abstract
OBJECTIVE—Treatment of ventricular tachycardia (VT) in coronary heart disease has to date been limited to palliative treatment with drugs or implantable defibrillators. The results of curative treatment with catheter ablation have proved disappointing because the complexity of the VT mechanism makes identification of the substrate using conventional mapping techniques difficult. The use of a mapping technology that may address some of these issues, and thus make possible a cure for VT with catheter ablation, is reported. PATIENTS AND INTERVENTION—The non-contact system, consisting of a multielectrode array catheter (MEA) and a computer mapping system, was used to map VT in 24 patients. Twenty two patients had structural heart disease, the remainder having "normal" left ventricles with either fasicular tachycardia or left ventricular ectopic tachycardia. RESULTS—Exit sites were demonstrated in 80 of 81 VT morphologies by the non-contact system, and complete VT circuits were traced in 17. In another 37 morphologies of VT 36 (30)% (mean (SD)) of the diastolic interval was identified. Thirty eight VT morphologies were ablated using 154 radiofrequency energy applications. Successful ablation was achieved by 77% of radiofrequency within diastolic activation identified by the non-contact system and was significantly more likely to ablate VT than radiofrequency at the VT exit, or remote from diastolic activation. Over a mean follow up of 1.5 years, 14 patients have had no recurrence of VT and only two target VTs have recurred. Five patients have had recurrence of either slower non-sustained, undocumented or fast non-target VT. Five patients have died, one from tamponade from a pre-existing temporary pacing wire, and four from causes unrelated to the procedure. CONCLUSION—The non-contact system can safely be used to map and ablate haemodynamically stable VT with low VT recurrence rates. It is yet to be established whether this system may be applied with equal success to patients with haemodynamically unstable VT. Keywords: ventricular tachycardia; mapping; ablation
Full Text
The Full Text of this article is available as a PDF (211.1 KB).
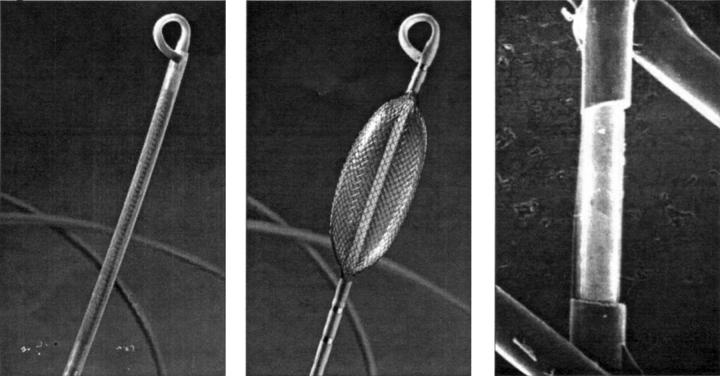
Figure 1 .
The non-contact mapping catheter with 9 French shaft (left), deployed 7.5 ml balloon with braided microelectrode array (centre), and micrograph of 0.025 inch long electrode created by removing a spot of insulation with a laser (right).
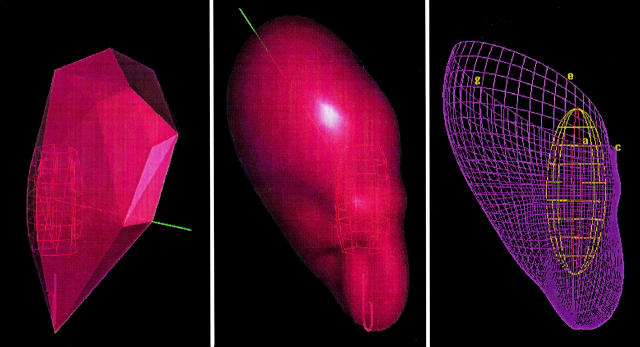
Figure 2 .
A contoured model of the chamber geometry is created by tracing the endocardial surface with a conventional roving catheter while the system tracks its position. The geometry is partially defined midway through the process in the left panel, completed and smoothed in the centre panel, and rendered in the right panel as an anatomically-contoured, wire-frame three dimensional model.
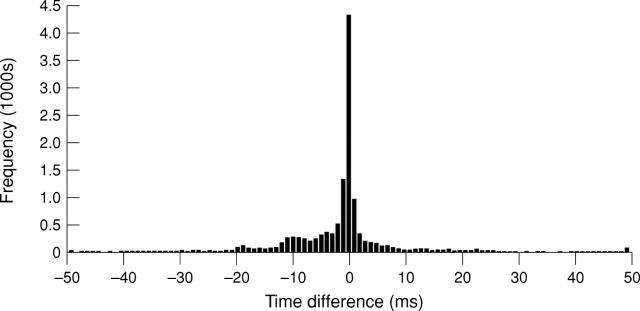
Figure 3 .
Timing difference between conventional contact electrograms and non-contact reconstructed electrograms recorded during sinus rhythm. A mean timing difference of −0.9 ms and a mean morphology cross correlation of 0.83 was demonstrated over a population of 31 487 recorded cardiac cycles. One half of the time differences were within 2 ms and 77% were within 10 ms.
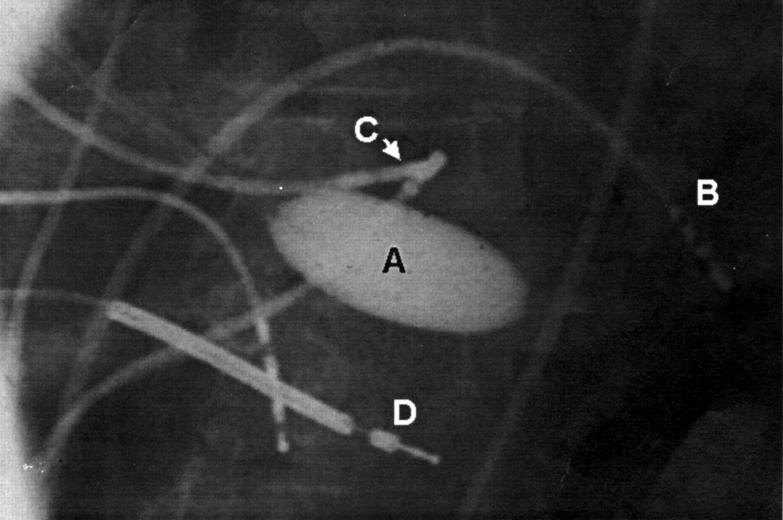
Figure 4 .
A posterio-anterior radiograph showing the non-contact mapping catheter with 7.5 ml of contrast medium/saline in the balloon, deployed in the left ventricle(A). Also seen in the left ventricle is a transeptal mapping catheter (B) and a retrograde transaortic mapping catheter (C). Other catheters are positioned in the right ventricular apex and coronary sinus. This patient also has an implantable defibrillator lead (D).
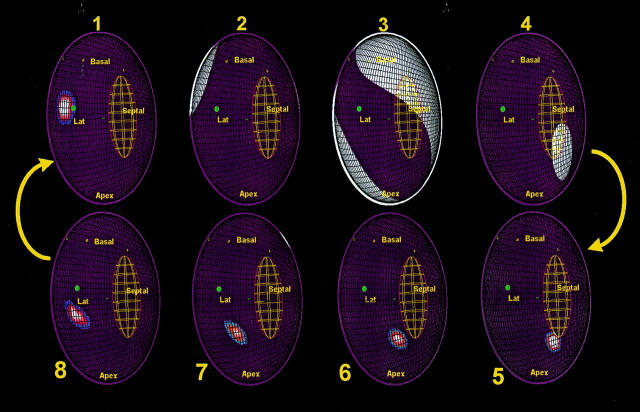
Figure 5 .
Activation maps recorded during VT in a patient with two VT morphologies using the same re-entry circuit in contrarotation. The virtual endocardium has been opened along the anterior septum so that the two edges are in continuity. Labels have been placed on sites identified using fluoroscopy as follows: Basal, left ventricle base; Apex, left ventricle apex; Septal, septum; Lat, lateral. Activation is indicated by white areas. The successful radiofrequency site is shown with a green dot. Activation reaches the exit of the diastolic component of the re-entry circuit (frame 1) before systolic activation of the left ventricle is seen (frames 2 to 4); note the sparing of the region of the diastolic pathway. The lines of conduction block that define this diastolic pathway are shown as green lines. The wavefront re-enters the diastolic pathway (frames 5 to 8) and progresses from the apicoseptum to exit at the basal-lateral end (frame 1).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonhoeffer P., Bonnet D., Piéchaud J. F., Stümper O., Aggoun Y., Villain E., Kachaner J., Sidi D. Coronary artery obstruction after the arterial switch operation for transposition of the great arteries in newborns. J Am Coll Cardiol. 1997 Jan;29(1):202–206. doi: 10.1016/s0735-1097(96)00433-0. [DOI] [PubMed] [Google Scholar]
- Derfus D. L., Pilkington T. C. Assessing the effect of uncertainty in intracavitary electrode position on endocardial potential estimates. IEEE Trans Biomed Eng. 1992 Jul;39(7):676–681. doi: 10.1109/10.142642. [DOI] [PubMed] [Google Scholar]
- El-Sherif N., Mehra R., Gough W. B., Zeiler R. H. Reentrant ventricular arrhythmias in the late myocardial infarction period. Interruption of reentrant circuits by cryothermal techniques. Circulation. 1983 Sep;68(3):644–656. doi: 10.1161/01.cir.68.3.644. [DOI] [PubMed] [Google Scholar]
- Gepstein L., Hayam G., Ben-Haim S. A. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation. 1997 Mar 18;95(6):1611–1622. doi: 10.1161/01.cir.95.6.1611. [DOI] [PubMed] [Google Scholar]
- Josephson M. E., Horowitz L. N., Farshidi A., Kastor J. A. Recurrent sustained ventricular tachycardia. 1. Mechanisms. Circulation. 1978 Mar;57(3):431–440. doi: 10.1161/01.cir.57.3.431. [DOI] [PubMed] [Google Scholar]
- Karagueuzian H. S., Fenoglio J. J., Jr, Weiss M. B., Wit A. L. Protracted ventricular tachcardia induced by premature stimulation of the canine heart after coronary artery occlusion and reperfusion. Circ Res. 1979 Jun;44(6):833–846. doi: 10.1161/01.res.44.6.833. [DOI] [PubMed] [Google Scholar]
- Khoury D. S., Rudy Y. A model study of volume conductor effects on endocardial and intracavitary potentials. Circ Res. 1992 Sep;71(3):511–525. doi: 10.1161/01.res.71.3.511. [DOI] [PubMed] [Google Scholar]
- Khoury D. S., Taccardi B., Lux R. L., Ershler P. R., Rudy Y. Reconstruction of endocardial potentials and activation sequences from intracavitary probe measurements. Localization of pacing sites and effects of myocardial structure. Circulation. 1995 Feb 1;91(3):845–863. doi: 10.1161/01.cir.91.3.845. [DOI] [PubMed] [Google Scholar]
- Morady F., Harvey M., Kalbfleisch S. J., el-Atassi R., Calkins H., Langberg J. J. Radiofrequency catheter ablation of ventricular tachycardia in patients with coronary artery disease. Circulation. 1993 Feb;87(2):363–372. doi: 10.1161/01.cir.87.2.363. [DOI] [PubMed] [Google Scholar]
- Peters N. S., Jackman W. M., Schilling R. J., Beatty G., Davies D. W. Images in cardiovascular medicine. Human left ventricular endocardial activation mapping using a novel noncontact catheter. Circulation. 1997 Mar 18;95(6):1658–1660. doi: 10.1161/01.cir.95.6.1658. [DOI] [PubMed] [Google Scholar]
- Roberts S. A., Viana M. A., Nazari J., Bauman J. L. Invasive and noninvasive methods to predict the long-term efficacy of amiodarone: a compilation of clinical observations using meta-analysis. Pacing Clin Electrophysiol. 1994 Oct;17(10):1590–1602. doi: 10.1111/j.1540-8159.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Roy D., Waxman H. L., Kienzle M. G., Buxton A. E., Marchlinski F. E., Josephson M. E. Clinical characteristics and long-term follow-up in 119 survivors of cardiac arrest: relation to inducibility at electrophysiologic testing. Am J Cardiol. 1983 Nov 1;52(8):969–974. doi: 10.1016/0002-9149(83)90514-3. [DOI] [PubMed] [Google Scholar]
- Schilling R. J., Peters N. S., Davies D. W. Simultaneous endocardial mapping in the human left ventricle using a noncontact catheter: comparison of contact and reconstructed electrograms during sinus rhythm. Circulation. 1998 Sep 1;98(9):887–898. doi: 10.1161/01.cir.98.9.887. [DOI] [PubMed] [Google Scholar]
- Stevenson W. G., Friedman P. L., Kocovic D., Sager P. T., Saxon L. A., Pavri B. Radiofrequency catheter ablation of ventricular tachycardia after myocardial infarction. Circulation. 1998 Jul 28;98(4):308–314. doi: 10.1161/01.cir.98.4.308. [DOI] [PubMed] [Google Scholar]
- Stevenson W. G., Khan H., Sager P., Saxon L. A., Middlekauff H. R., Natterson P. D., Wiener I. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993 Oct;88(4 Pt 1):1647–1670. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- Swerdlow C. D., Winkle R. A., Mason J. W. Determinants of survival in patients with ventricular tachyarrhythmias. N Engl J Med. 1983 Jun 16;308(24):1436–1442. doi: 10.1056/NEJM198306163082402. [DOI] [PubMed] [Google Scholar]
- Taccardi B., Arisi G., Macchi E., Baruffi S., Spaggiari S. A new intracavitary probe for detecting the site of origin of ectopic ventricular beats during one cardiac cycle. Circulation. 1987 Jan;75(1):272–281. doi: 10.1161/01.cir.75.1.272. [DOI] [PubMed] [Google Scholar]
- de Bakker J. M., Coronel R., Tasseron S., Wilde A. A., Opthof T., Janse M. J., van Capelle F. J., Becker A. E., Jambroes G. Ventricular tachycardia in the infarcted, Langendorff-perfused human heart: role of the arrangement of surviving cardiac fibers. J Am Coll Cardiol. 1990 Jun;15(7):1594–1607. doi: 10.1016/0735-1097(90)92832-m. [DOI] [PubMed] [Google Scholar]