Abstract
OBJECTIVE—Angiotensin II (AII) and aldosterone are not always fully suppressed during chronic angiotensin converting enzyme (ACE) inhibitor treatment. In congestive heart failure (CHF) such failure of hormonal suppression is associated with increased mortality. This study examined how common AII and aldosterone increases are observed during routine clinical practice. PATIENTS AND METHODS—91 patients with symptomatic (mean New York Heart Association class 2.7) CHF (mean (SD) left ventricular ejection fraction 29.9 (8)%, range 9-46%) were studied 4-6 hours after ACE inhibitor dosing. A representative range of ACE inhibitors (enalapril, lisinopril, captopril, perindopril, and fosinopril) was examined. RESULTS—Supine measurements showed a wide range of AII (10.5 (25.5) pg/ml), aldosterone (130.8 (136) pg/ml), and serum ACE (12.1 (13.3) EU/l; excludes captopril data) concentrations on diuretics. AII concentrations > 10 pg/ml were seen in 15% of patients, and aldosterone concentrations > 144 pg/ml were seen in 38% of patients. AII concentrations were significantly correlated (p < 0.001) with ACE but not with aldosterone concentrations. Aldosterone concentrations were not significantly correlated with ACE concentrations. CONCLUSIONS—AII "reactivation" occurred in 15% and failure of aldosterone suppression in 38% of routine CHF patients taking ACE inhibitor treatment. AII "reactivation" was associated with both low and high levels of ACE activity, which suggests that multiple different mechanisms are at play. In patients with high plasma ACE concentrations, non-compliance should be considered along with inadequate dose titration. In patients with low plasma ACE and high AII concentrations, non-ACE mediated production of AII may be operative. Raised aldosterone concentrations appear to be more common than AII "reactivation". It is important to establish the cause of detectable or increased AII concentrations in a heart failure patient treated with an ACE inhibitor. The measurement of serum ACE may help to identify the likely cause as poor compliance or inadequate dose. Keywords: heart failure; hormone suppression; angiotensin II; aldosterone; angiotensin converting enzyme inhibitors; compliance
Full Text
The Full Text of this article is available as a PDF (123.6 KB).
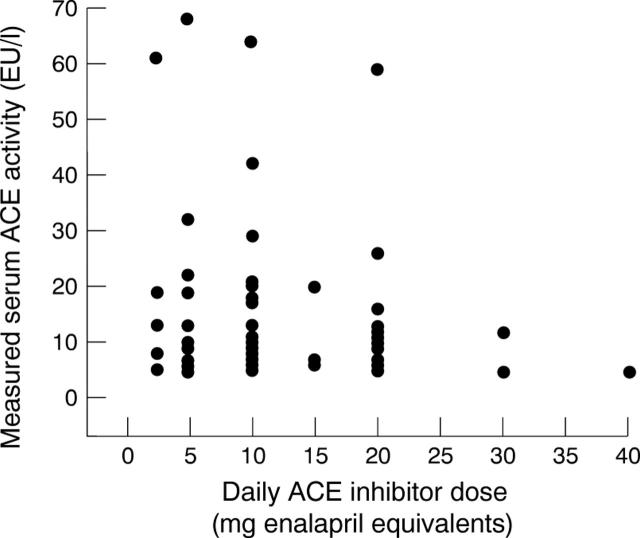
Figure 1 .
Relation between measured ACE activity and normalised ACE inhibitor dose (excluding those patients receiving oral captopril).
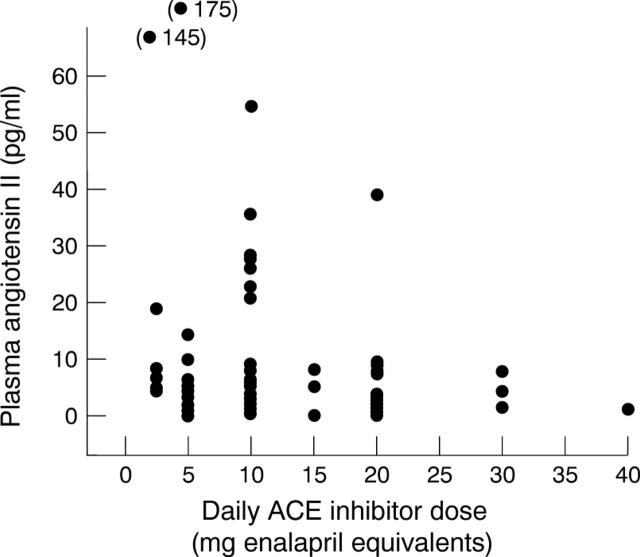
Figure 2 .
Relation between measured AII and normalised ACE inhibitor dose.
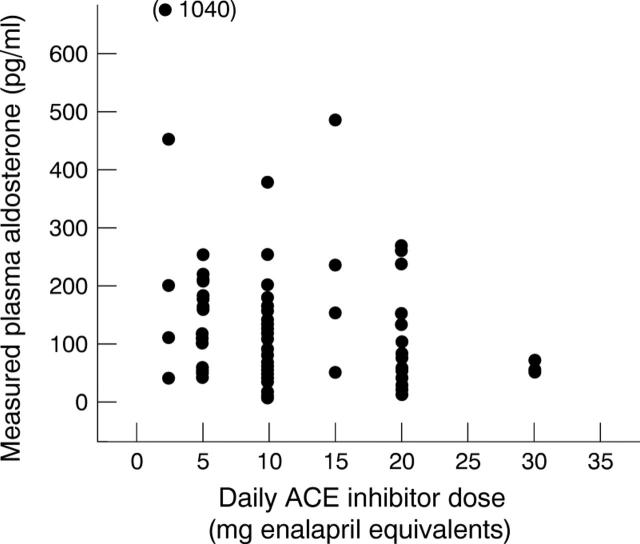
Figure 3 .
Relation between measured aldosterone and normalised ACE inhibitor dose.
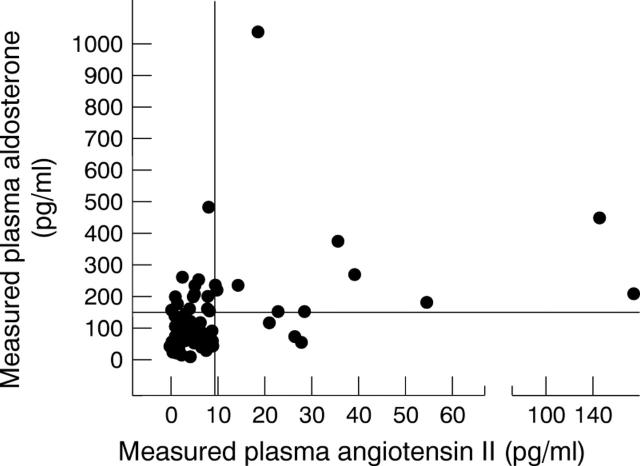
Figure 4 .
Relation between measured aldosterone and measured AII in same sample.
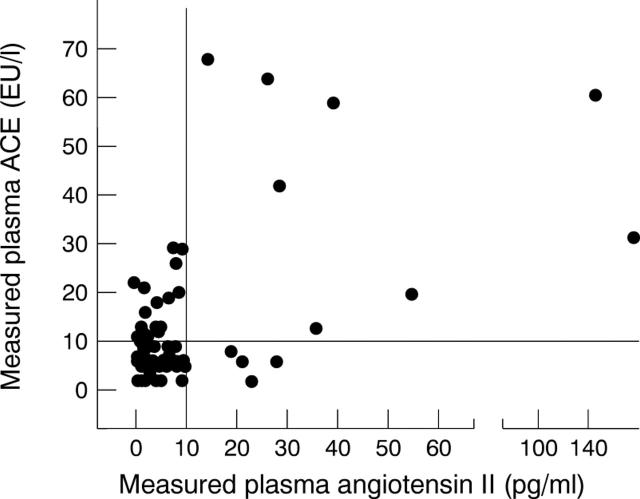
Figure 5 .
Relation between measured AII and ACE activity. There was a significant (r = 0.75, p < 0.001) correlation.
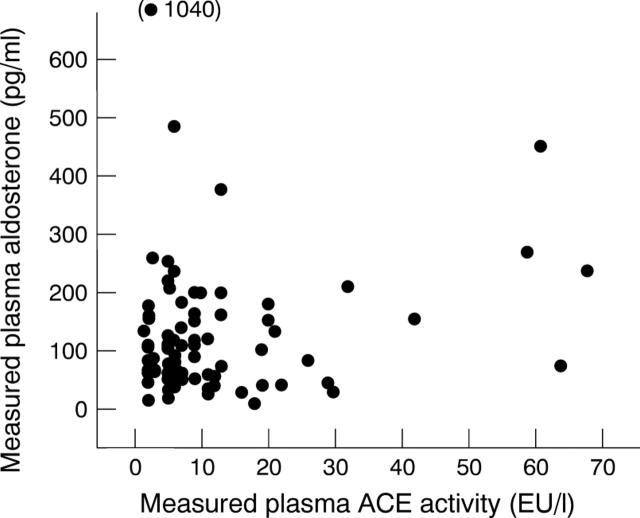
Figure 6 .
Relation between measured aldosterone and ACE activity. No significant correlation found.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belz G. G., Kirch W., Kleinbloesem C. H. Angiotensin-converting enzyme inhibitors. Relationship between pharmacodynamics and pharmacokinetics. Clin Pharmacokinet. 1988 Nov;15(5):295–318. doi: 10.2165/00003088-198815050-00003. [DOI] [PubMed] [Google Scholar]
- Davidson N. C., Coutie W. J., Webb D. J., Struthers A. D. Hormonal and renal differences between low dose and high dose angiotensin converting enzyme inhibitor treatment in patients with chronic heart failure. Heart. 1996 Jun;75(6):576–581. [PMC free article] [PubMed] [Google Scholar]
- Fogarty Y., Fraser C. G., Browning M. C. Intra- and inter-individual variation of serum angiotensin converting enzyme: clinical implications. Ann Clin Biochem. 1989 Mar;26(Pt 2):201–202. doi: 10.1177/000456328902600224. [DOI] [PubMed] [Google Scholar]
- Garg R., Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995 May 10;273(18):1450–1456. [PubMed] [Google Scholar]
- Juillerat L., Nussberger J., Ménard J., Mooser V., Christen Y., Waeber B., Graf P., Brunner H. R. Determinants of angiotensin II generation during converting enzyme inhibition. Hypertension. 1990 Nov;16(5):564–572. doi: 10.1161/01.hyp.16.5.564. [DOI] [PubMed] [Google Scholar]
- MacFadyen R. J., Struthers A. D. The practical assessment of compliance with ACE-inhibitor therapy--a novel approach. J Cardiovasc Pharmacol. 1997 Jan;29(1):119–124. doi: 10.1097/00005344-199701000-00018. [DOI] [PubMed] [Google Scholar]
- Morton J. J., Webb D. J. Measurement of plasma angiotensin II. Clin Sci (Lond) 1985 Apr;68(4):483–484. doi: 10.1042/cs0680483. [DOI] [PubMed] [Google Scholar]
- Nussberger J., Waeber G., Waeber B., Bidiville J., Brunner H. R. Plasma angiotensin-(1-8) octapeptide measurement to assess acute angiotensin-converting enzyme inhibition with captopril administered parenterally to normal subjects. J Cardiovasc Pharmacol. 1988 Jun;11(6):716–721. doi: 10.1097/00005344-198806000-00013. [DOI] [PubMed] [Google Scholar]
- Opasich C., Febo O., Riccardi P. G., Traversi E., Forni G., Pinna G., Pozzoli M., Riccardi R., Mortara A., Sanarico M. Concomitant factors of decompensation in chronic heart failure. Am J Cardiol. 1996 Aug 1;78(3):354–357. doi: 10.1016/s0002-9149(96)00294-9. [DOI] [PubMed] [Google Scholar]
- Packer M. New concepts in the pathophysiology of heart failure: beneficial and deleterious interaction of endogenous haemodynamic and neurohormonal mechanisms. J Intern Med. 1996 Apr;239(4):327–333. doi: 10.1046/j.1365-2796.1996.463796000.x. [DOI] [PubMed] [Google Scholar]
- Rich M. W., Vinson J. M., Sperry J. C., Shah A. S., Spinner L. R., Chung M. K., Davila-Roman V. Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. J Gen Intern Med. 1993 Nov;8(11):585–590. doi: 10.1007/BF02599709. [DOI] [PubMed] [Google Scholar]
- Sigurdsson A., Held P., Andersson G., Swedberg K. Enalaprilat in acute myocardial infarction: tolerability and effects on the renin-angiotensin system. Int J Cardiol. 1991 Oct;33(1):115–124. doi: 10.1016/0167-5273(91)90159-m. [DOI] [PubMed] [Google Scholar]
- Sigurdsson A., Swedberg K. The role of neurohormonal activation in chronic heart failure and postmyocardial infarction. Am Heart J. 1996 Jul;132(1 Pt 2 SU):229–234. [PubMed] [Google Scholar]
- Swedberg K., Eneroth P., Kjekshus J., Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990 Nov;82(5):1730–1736. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]