Abstract
OBJECTIVE—To investigate whether autonomic nervous activity is involved in the recurrence of spontaneous coronary spasm in variant angina. DESIGN—Retrospective analysis. SETTING—Cardiology department of a university hospital. PATIENTS—18 patients with variant angina were divided into single attack group (SA; nine patients) and multiple attack group (MA; nine patients) according to the frequency of ischaemic episodes with ST segment elevation during 24 hour Holter monitoring. METHODS—Heart rate variability indices were calculated using MemCalc method, which is a combination of the maximum entropy method for spectral analysis and the non-linear least squares method for fitting analysis, at 30 second intervals for 30 second periods, from 40 minutes before the attack to 30 minutes after the attack. High frequency (HF; 0.04-0.15 Hz) was defined as a marker of parasympathetic activity, and the ratio of low frequency (LF; 0.15-0.40 Hz) to high frequency (LF/HF) as an indicator of sympathetic activity. The averaged value during the 40 to 30 minute period before an attack was defined as the baseline. RESULTS—Compared with baseline, the HF component decreased in both groups at two minutes before the attack (p < 0.01), and the LF/HF ratio decreased at three minutes before the attack (p < 0.01). The baseline LF/HF was lower in the MA group than in the SA group (p < 0.01). CONCLUSIONS—A reduction of sympathetic activity may play a key role in determining the recurrence of transient ischaemic events caused by spontaneous coronary spasm in patients with variant angina. Keywords: sympathetic activity; recurrence of coronary spasm; MemCalc method; variant angina
Full Text
The Full Text of this article is available as a PDF (274.7 KB).
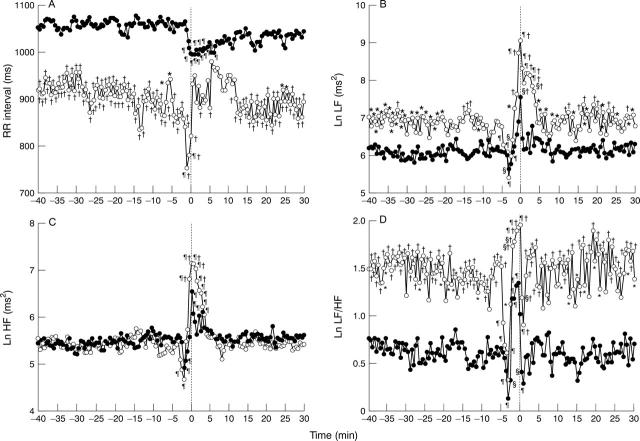
Figure 1 .
Graphs showing RR interval (A), LF (B), HF (C) and LF/HF ratio (D) in the single attack (SA) group (∘) and the multiple attack (MA) group (•) from 40 minutes before an attack to 30 minutes after an attack. For clarity, SD bars are not shown. Baseline = averaged value during the 40 to 30 minute period before an attack. The averaged RR interval in the MA group was significantly higher than in the SA group (p < 0.01). In both groups, HF significantly decreased compared with baseline at two minutes before an attack, while LF and LF/HF were significantly reduced compared with baseline at three minutes before an attack. For LF and LF/HF, the MA group showed significantly lower values than the SA group before and after the attack (p < 0.01, respectively). *p < 0.05 v MA group; †p < 0.01 v MA group; §p < 0.05 v baseline; ¶p < 0.01 v baseline. LF, low frequency component (0.04-0.15 Hz); HF, high frequency component (0.15-0.40 Hz).
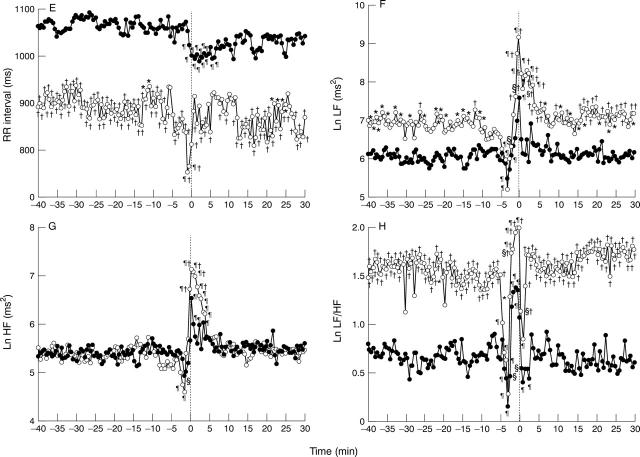
Figure 2 .
Graphs showing RR interval (E), LF (F), HF (G), and LF/HF ratio (H) in the single attack (SA) group (∘) and the multiple attack (MA) group (•) with ST segment elevation in the modified lead II of the Holter ECG from 40 minutes before an attack to 30 minutes after an attack. For clarity, SD bars are not shown. Baseline = averaged value during the 40 to 30 minute period before an attack. The results are similar to those of fig 1. *p < 0.05 v MA group; †p < 0.01 v MA group; §p < 0.05 v baseline; ¶p < 0.01 v baseline. LF, low frequency component (0.04-0.15 Hz); HF, high frequency component (0.15-0.40 Hz).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airaksinen K. E., Ikäheimo M. J., Huikuri H. V., Linnaluoto M. K., Takkunen J. T. Responses of heart rate variability to coronary occlusion during coronary angioplasty. Am J Cardiol. 1993 Nov 1;72(14):1026–1030. doi: 10.1016/0002-9149(93)90857-9. [DOI] [PubMed] [Google Scholar]
- Akselrod S., Gordon D., Ubel F. A., Shannon D. C., Berger A. C., Cohen R. J. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981 Jul 10;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Bernardi L., Leuzzi S., Radaelli A., Passino C., Johnston J. A., Sleight P. Low-frequency spontaneous fluctuations of R-R interval and blood pressure in conscious humans: a baroreceptor or central phenomenon? Clin Sci (Lond) 1994 Dec;87(6):649–654. doi: 10.1042/cs0870649. [DOI] [PubMed] [Google Scholar]
- Bigger J. T., Jr, Fleiss J. L., Steinman R. C., Rolnitzky L. M., Kleiger R. E., Rottman J. N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992 Jan;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Chierchia S., Davies G., Berkenboom G., Crea F., Crean P., Maseri A. alpha-Adrenergic receptors and coronary spasm: an elusive link. Circulation. 1984 Jan;69(1):8–14. doi: 10.1161/01.cir.69.1.8. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Hillis L. D., Braunwald E. Coronary-artery spasm. N Engl J Med. 1978 Sep 28;299(13):695–702. doi: 10.1056/NEJM197809282991305. [DOI] [PubMed] [Google Scholar]
- Jaffe R. S., Fung D. L., Behrman K. H. Optimal frequency ranges for extracting information on autonomic activity from the heart rate spectrogram. J Auton Nerv Syst. 1994 Jan-Feb;46(1-2):37–46. doi: 10.1016/0165-1838(94)90142-2. [DOI] [PubMed] [Google Scholar]
- King M. J., Zir L. M., Kaltman A. J., Fox A. C. Variant angina associated with angiographically demonstrated coronary artery spasm and REM sleep. Am J Med Sci. 1973 May;265(5):419–422. doi: 10.1097/00000441-197305000-00009. [DOI] [PubMed] [Google Scholar]
- Lanza G. A., Pedrotti P., Pasceri V., Lucente M., Crea F., Maseri A. Autonomic changes associated with spontaneous coronary spasm in patients with variant angina. J Am Coll Cardiol. 1996 Nov 1;28(5):1249–1256. doi: 10.1016/S0735-1097(96)00309-9. [DOI] [PubMed] [Google Scholar]
- Luria M. H., Sapoznikov D., Gilon D., Zahger D., Weinstein J. M., Weiss A. T., Gotsman M. S. Early heart rate variability alterations after acute myocardial infarction. Am Heart J. 1993 Mar;125(3):676–681. doi: 10.1016/0002-8703(93)90157-5. [DOI] [PubMed] [Google Scholar]
- Malliani A., Pagani M., Lombardi F., Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991 Aug;84(2):482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Maseri A., Severi S., Nes M. D., L'Abbate A., Chierchia S., Marzilli M., Ballestra A. M., Parodi O., Biagini A., Distante A. "Variant" angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia. Pathogenetic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol. 1978 Dec;42(6):1019–1035. doi: 10.1016/0002-9149(78)90691-4. [DOI] [PubMed] [Google Scholar]
- Miwa K., Goto M., Lee J. D., Matsuyama F., Shimizu H., Kato T., Hara A., Nakamura T. Supersensitivity of coronary arteries in variant angina to spasm induced by intracoronary acetylcholine. Am J Cardiol. 1988 Jan 1;61(1):77–82. doi: 10.1016/0002-9149(88)91308-2. [DOI] [PubMed] [Google Scholar]
- Nademanee K., Intarachot V., Josephson M. A., Singh B. N. Circadian variation in occurrence of transient overt and silent myocardial ischemia in chronic stable angina and comparison with Prinzmetal angina in men. Am J Cardiol. 1987 Sep 1;60(7):494–498. doi: 10.1016/0002-9149(87)90292-x. [DOI] [PubMed] [Google Scholar]
- Nowlin J. B., Troyer W. G., Jr, Collins W. S., Silverman G., Nichols C. R., McIntosh H. D., Estes E. H., Jr, Bogdonoff M. D. The association of nocturnal angina pectoris with dreaming. Ann Intern Med. 1965 Dec;63(6):1040–1046. doi: 10.7326/0003-4819-63-6-1040. [DOI] [PubMed] [Google Scholar]
- PRINZMETAL M., KENNAMER R., MERLISS R., WADA T., BOR N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959 Sep;27:375–388. doi: 10.1016/0002-9343(59)90003-8. [DOI] [PubMed] [Google Scholar]
- Pagani M., Lombardi F., Guzzetti S., Rimoldi O., Furlan R., Pizzinelli P., Sandrone G., Malfatto G., Dell'Orto S., Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986 Aug;59(2):178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pinna G. D., Maestri R., Di Cesare A., Colombo R., Minuco G. The accuracy of power-spectrum analysis of heart-rate variability from annotated RR lists generated by Holter systems. Physiol Meas. 1994 May;15(2):163–179. doi: 10.1088/0967-3334/15/2/006. [DOI] [PubMed] [Google Scholar]
- Pomeranz B., Macaulay R. J., Caudill M. A., Kutz I., Adam D., Gordon D., Kilborn K. M., Barger A. C., Shannon D. C., Cohen R. J. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985 Jan;248(1 Pt 2):H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Rizzoni D., Castellano M., Beschi M., Muiesan M. L., Bettoni G., Porteri E., Agabiti-Rosei E. Plasma norepinephrine and spectral analysis of the heart rate during cardiopulmonary receptor stimulation in normal and hypertensive subjects. J Hypertens Suppl. 1991 Dec;9(6):S84–S85. [PubMed] [Google Scholar]
- Robertson R. M., Bernard Y., Robertson D. Arterial and coronary sinus catecholamines in the course of spontaneous coronary artery spasm. Am Heart J. 1983 Jun;105(6):901–906. doi: 10.1016/0002-8703(83)90387-3. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Ohtomo N., Tanaka Y., Tanaka G., Yamakoshi K., Terachi S., Shimamoto K., Nakagawa M., Satoh S., Kuroda S. New technique for time series analysis combining the maximum entropy method and non-linear least squares method: its value in heart rate variability analysis. Med Biol Eng Comput. 1997 Jul;35(4):318–322. doi: 10.1007/BF02534083. [DOI] [PubMed] [Google Scholar]
- Somers V. K., Dyken M. E., Mark A. L., Abboud F. M. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993 Feb 4;328(5):303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- Specchia G., de Servi S., Falcone C., Bramucci E., Angoli L., Mussini A., Marinoni G. P., Montemartini C., Bobba P. Coronary arterial spasm as a cause of exercise-induced ST-segment elevation in patients with variant angina. Circulation. 1979 May;59(5):948–954. doi: 10.1161/01.cir.59.5.948. [DOI] [PubMed] [Google Scholar]
- Takano H., Nakamura T., Satou T., Umetani K., Watanabe A., Ishihara T., Mochizuki S., Kimura H., Honma H., Ikeda Y. Regional myocardial sympathetic dysinnervation in patients with coronary vasospasm. Am J Cardiol. 1995 Feb 15;75(5):324–329. doi: 10.1016/s0002-9149(99)80547-5. [DOI] [PubMed] [Google Scholar]
- Vila J., Palacios F., Presedo J., Fernández-Delgado M., Felix P., Barro S. Time-frequency analysis of heart-rate variability. IEEE Eng Med Biol Mag. 1997 Sep-Oct;16(5):119–126. doi: 10.1109/51.620503. [DOI] [PubMed] [Google Scholar]
- Waters D. D., Miller D. D., Bouchard A., Bosch X., Theroux P. Circadian variation in variant angina. Am J Cardiol. 1984 Jul 1;54(1):61–64. doi: 10.1016/0002-9149(84)90304-7. [DOI] [PubMed] [Google Scholar]
- Yasue H., Horio Y., Nakamura N., Fujii H., Imoto N., Sonoda R., Kugiyama K., Obata K., Morikami Y., Kimura T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986 Nov;74(5):955–963. doi: 10.1161/01.cir.74.5.955. [DOI] [PubMed] [Google Scholar]
- Yasue H., Touyama M., Kato H., Tanaka S., Akiyama F. Prinzmetal's variant form of angina as a manifestation of alpha-adrenergic receptor-mediated coronary artery spasm: documentation by coronary arteriography. Am Heart J. 1976 Feb;91(2):148–155. doi: 10.1016/s0002-8703(76)80568-6. [DOI] [PubMed] [Google Scholar]
- Yasue H., Touyama M., Kato H., Tanaka S., Akiyama F. Prinzmetal's variant form of angina as a manifestation of alpha-adrenergic receptor-mediated coronary artery spasm: documentation by coronary arteriography. Am Heart J. 1976 Feb;91(2):148–155. doi: 10.1016/s0002-8703(76)80568-6. [DOI] [PubMed] [Google Scholar]
- Yeragani V. K., Srinivasan K., Vempati S., Pohl R., Balon R. Fractal dimension of heart rate time series: an effective measure of autonomic function. J Appl Physiol (1985) 1993 Dec;75(6):2429–2438. doi: 10.1152/jappl.1993.75.6.2429. [DOI] [PubMed] [Google Scholar]
- Yoshio H., Shimizu M., Sugihara N., Kita Y., Shimizu K., Minagawa F., Nakabayashi H., Takeda R. Assessment of autonomic nervous activity by heart rate spectral analysis in patients with variant angina. Am Heart J. 1993 Feb;125(2 Pt 1):324–329. doi: 10.1016/0002-8703(93)90007-v. [DOI] [PubMed] [Google Scholar]