Abstract
OBJECTIVE—To address a potential role for p53, Bcl2 associated protein X (Bax), and apoptosis in the processes associated with cell turnover during cystic medial degeneration (CMD) of the aorta. METHODS—Histochemical, immunohistochemical, biochemical, and morphometric methods were used to assess the presence and distribution of p53 immunoreactivity (p53-IR) and Bax immunoreactivity (Bax-IR), as well as the presence of apoptosis and tissue repair processes. RESULTS—Immunohistochemical staining disclosed evidence for p53-IR in all specimens in 26.1 (11.5)% of vascular smooth muscle cells (VSMCs) (controls 0.8 (1.3)%; p < 0.001). Bax-IR was present in all specimens in 10 (5.4)% of medial cells (controls 0.3 (0.5)%; p < 0.001). Medial VSMCs (α-actin positive) with cytoplasmic staining for an apoptosis specific protein (c-jun/ASP) were present in 20/20 specimens (0.7 (0.6)% of VSMCs, controls 0%, p < 0.001), whereas terminal deoxynucleotidyl transferase mediated dUTP-biotin nick end labeling (TUNEL) positive VSMCs were present in 17/20 specimens (1 (1.5)% of VSMCs, controls 0%, p < 0.001). The presence of apoptosis was confirmed by electron microscopy and the demonstration of oligonucleosomal DNA fragments after agarose gel electrophoresis. As shown by double labeling and investigation of serial sections, p53-IR, Bax-IR, c-jun/ASP-IR, and positive TUNEL labeling localised to the same compartments of the aortic media, raising a possible role for p53 and Bax in the triggering of apoptosis of VSMC during CMD. MIB1/Ki-67 positive medial VSMCs (α-actin positive) and mesenchymal cells (vimentin positive) were present in all specimens (2.5 (2.8)% of medial cells; controls 0.3 (0.9)%, p < 0.001) mainly in the region around the vasa vasorum, indicating that cell regeneration during CMD may originate mainly from the mesenchyme surrounding the vasa vasorum. CONCLUSION—This study shows that the formal pathogenesis of CMD is characterised by p53 accumulation, Bax upregulation, cell death by apoptosis, and cell regeneration. Nevertheless, the precise stimuli of p53 activation and Bax upregulation as well as the role of p53 and apoptosis in the dissection process itself remain elusive. Keywords: mucoid cystic medial degeneration of the aorta; p53 accumulation; apoptosis; cell proliferation
Full Text
The Full Text of this article is available as a PDF (212.3 KB).
Figure 1 .
(A) Low power magnification from a p53 stained section of a segment of a human aorta with CMD. Shown is an area at some distance from the dissection plane with only minor degrees of structural changes. There are numerous nuclei in a haphazard distribution displaying a positive staining for p53 (brown reaction product in the nuclei). (B) Higher power magnification of the region which is marked by an arrow on A. Note the p53 positive nucleus indicated by the arrow displays the typical nuclear morphology of apoptosis—for example, chromatin condensation and fragmentation. (C) Low power magnification of a p53 stained section of a segment of a human aorta with CMD showing the plane of dissection (upper half) and its immediate vicinity (lower half). There are numerous p53 positive nuclei in a homogenous distribution. Inset: double immunostaining for p53 (brown reaction product, ABC method) and α-actin (blue reaction product, APAAP method) showing a medial VSMC with nuclear p53 accumulation. (D) Low power magnification of a TUNEL stained section of a segment of a human aorta with CMD showing the plane of dissection (right side). There are numerous nuclear fragments exhibiting a positive TUNEL stain located in the immediate vicinity of the dissection plane.
Figure 2 .

(Top) Low power magnification from a Bax stained section of human aorta with only minor degrees of CMD showing two medial cells with cytoplasmic Bax positivity (arrows, reddish brown reaction product). (B) High power magnification from a Bax/p53 immuno double labeling showing an area with advanced CMD. In a region with pseudocystic degeneration there are three cells showing cytoplasmic Bax positivity (red reaction product) and simultaneous nuclear p53 staining (dark brown reaction product).
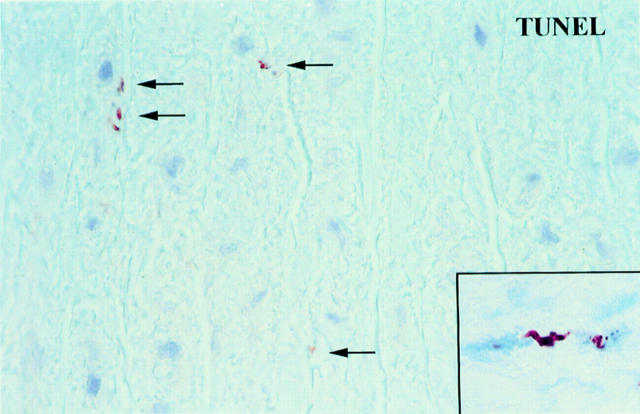
Figure 3 .
High power magnification from a TUNEL stained section of a segment of a human aorta with CMD at some distance from the dissection plane. There are several small TUNEL positive particles (arrows) probably corresponding to the apoptotic bodies shown in fig 6C. Inset: double labeling showing medial VSMCs with a positive TUNEL stain in the nucleus (brown reaction product) and cytoplasmic staining for α-actin (blue reaction product, APAAP method). One TUNEL positive nucleus shows the typical fragmentation which is a morphological feature of apoptosis.
Figure 4 .
(A) H&E staining of a representative part of aortic control tissue used for DNA fragmentation analysis. There are no structural alterations in the media. The nuclei are oriented in a parallel fashion to the elastic fibres. (B) H&E staining of a representative part of the aortic tissue from the patient with an aneurysm of the ascending aorta used for DNA fragmentation analysis showing the typical alterations of CMD—for example, small pseudocysts filled with basophilic amorphous material (arrows) and focal loss of nuclei (asterisks). Signs of inflammation in the media were absent. In addition, despite the focal absence of cell nuclei (asterisks), the total cell count in B is higher than in A.
Figure 5 .

Agarose gel electrophoresis of DNA isolated from a control patient and a patient with CMD. Lane 1, control patient; lane 2, patient with CMD. In lane 2 there are oligonucleosomal DNA fragments indicating cell death by apoptosis. In contrast, in the control tissue oligonucleosomal DNA fragments were absent. Top of lane 1—total amount of DNA brought into the gel.
Figure 6 .
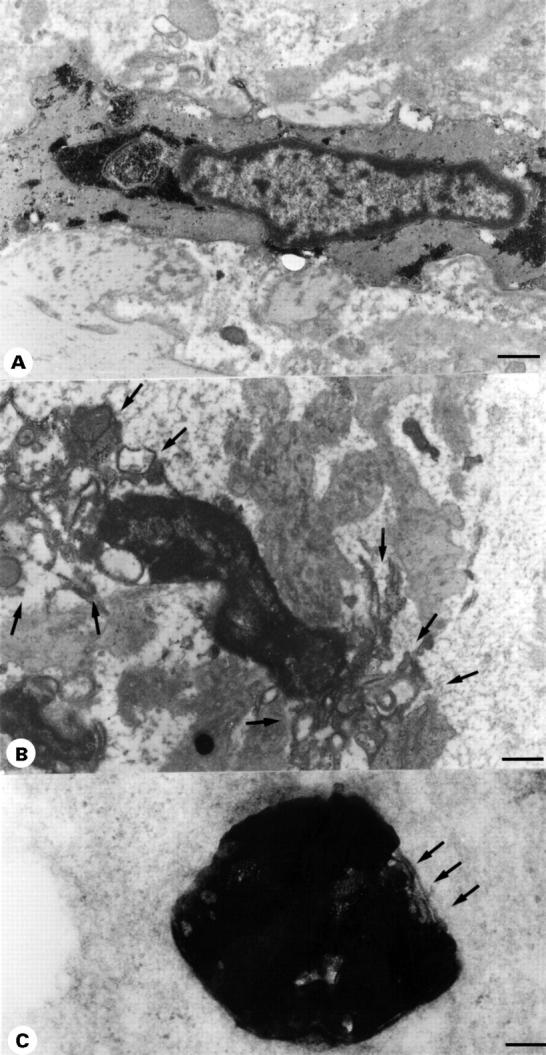
(A) Transmission electron micrograph of a normal VSCM from a patient with an aneurysm of the ascending thoracic aorta with CMD. Cellular organelles and the nucleus are intact and large amounts of glycogen are present. Scale bar 5 µm. (B) Transmission electron micrograph of a VSMC from the same patient displaying the typical ultrastructural features of the early stages of apoptosis—for example, membrane blebbing (arrows), dissolution of the cytoplasm, as well as condensation and margination of the chromatin. For comparison no such changes are present in A. Scale bar 5 µm. (C) In the aortic media of the same patient numerous typical apoptotic bodies representing the late stage of programmed cell death were present. They contain an intensely osmophilic amorphous material probably representing a fragment of a condensed electron dense nucleus and are membrane bound (arrows). Scale bar 2 µm.
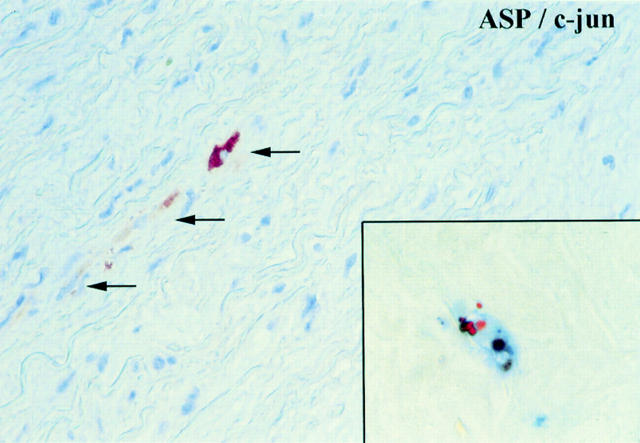
Figure 7 .
Section of a human aorta with CMD showing few medial VSMCs with a positive cytoplasmic staining for c-jun/ASP (brown reaction product). Inset: double labeling showing a medial VSMCs with a positive TUNEL stain in the nucleus (brown reaction product) and cytoplasmic staining for c-jun/ASP (blue reaction product, APAAP method). The TUNEL positive nucleus shows a fragmentation which is a typical morphological sign of apoptosis.
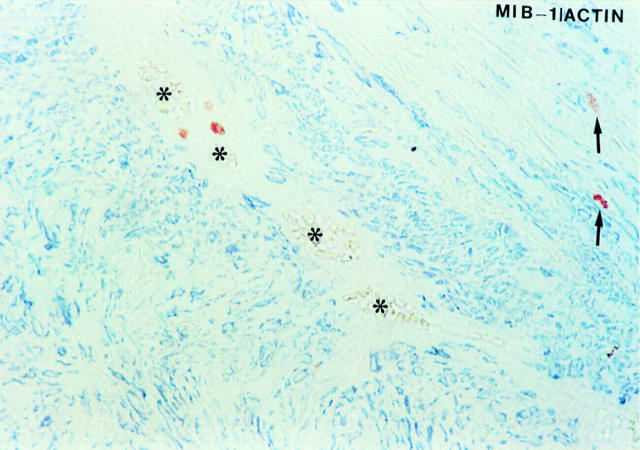
Figure 8 .
Double immunostaining for MIB1/Ki-67 (brown reaction product, ABC method) and α-actin (blue reaction product, APAAP method) showing three MIB1/Ki-67 positive/α-actin negative cells in the regions around the vasa vasorum (asterisks) and two MIB1/Ki-67 positive/α-actin positive VSMCs in the media (arrows).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Kiritsy C. P., Lynch S. E. p53 expression during normal tissue regeneration in response to acute cutaneous injury in swine. J Clin Invest. 1994 May;93(5):2206–2214. doi: 10.1172/JCI117217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Marcantonio E. E. The extracellular matrix as a cell cycle control element in atherosclerosis and restenosis. J Clin Invest. 1996 Dec 1;98(11):2436–2439. doi: 10.1172/JCI119059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Littlewood T. D., Schwartz S. M., Weissberg P. L. Increased sensitivity of human vascular smooth muscle cells from atherosclerotic plaques to p53-mediated apoptosis. Circ Res. 1997 Oct;81(4):591–599. doi: 10.1161/01.res.81.4.591. [DOI] [PubMed] [Google Scholar]
- Björkerud S., Björkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol. 1996 Aug;149(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Cheng W., Li B., Kajstura J., Li P., Wolin M. S., Sonnenblick E. H., Hintze T. H., Olivetti G., Anversa P. Stretch-induced programmed myocyte cell death. J Clin Invest. 1995 Nov;96(5):2247–2259. doi: 10.1172/JCI118280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Treasure T., Richardson P. D. The pathogenesis of spontaneous arterial dissection. Heart. 1996 May;75(5):434–435. doi: 10.1136/hrt.75.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSanctis R. W., Doroghazi R. M., Austen W. G., Buckley M. J. Aortic dissection. N Engl J Med. 1987 Oct 22;317(17):1060–1067. doi: 10.1056/NEJM198710223171705. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Li L., Schlueter C., Duchrow M., Wohlenberg C., Gerlach C., Stahmer I., Kloth S., Brandt E., Flad H. D. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991 Apr;138(4):867–873. [PMC free article] [PubMed] [Google Scholar]
- Gottlieb T. M., Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996 Jun 7;1287(2-3):77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Milner A. E., Mustoe T., Johnson G. D., Owen D., Grant M. L., Gregory C. D. A novel protein expressed in mammalian cells undergoing apoptosis. Exp Cell Res. 1995 Jun;218(2):439–451. doi: 10.1006/excr.1995.1177. [DOI] [PubMed] [Google Scholar]
- Haunstetter A., Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998 Jun 15;82(11):1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- Ihling C., Haendeler J., Menzel G., Hess R. D., Fraedrich G., Schaefer H. E., Zeiher A. M. Co-expression of p53 and MDM2 in human atherosclerosis: implications for the regulation of cellularity of atherosclerotic lesions. J Pathol. 1998 Jul;185(3):303–312. doi: 10.1002/(SICI)1096-9896(199807)185:3<303::AID-PATH106>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ihling C., Menzel G., Wellens E., Mönting J. S., Schaefer H. E., Zeiher A. M. Topographical association between the cyclin-dependent kinases inhibitor P21, p53 accumulation, and cellular proliferation in human atherosclerotic tissue. Arterioscler Thromb Vasc Biol. 1997 Oct;17(10):2218–2224. doi: 10.1161/01.atv.17.10.2218. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Kearney M., Bortman S., Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995 Jun 1;91(11):2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- James T. N. Normal and abnormal consequences of apoptosis in the human heart. From postnatal morphogenesis to paroxysmal arrhythmias. Circulation. 1994 Jul;90(1):556–573. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kerr J. F. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971 Sep;105(1):13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- Kockx M. M., De Meyer G. R., Muhring J., Jacob W., Bult H., Herman A. G. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998 Jun 16;97(23):2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- Kockx M. M., Muhring J., Knaapen M. W., de Meyer G. R. RNA synthesis and splicing interferes with DNA in situ end labeling techniques used to detect apoptosis. Am J Pathol. 1998 Apr;152(4):885–888. [PMC free article] [PubMed] [Google Scholar]
- Kuwano K., Hagimoto N., Nomoto Y., Kawasaki M., Kunitake R., Fujita M., Miyazaki H., Hara N. P53 and p21 (Waf1/Cip1) mRNA expression associated with DNA damage and repair in acute immune complex alveolitis in mice. Lab Invest. 1997 Feb;76(2):161–169. [PubMed] [Google Scholar]
- Kuwano K., Kunitake R., Kawasaki M., Nomoto Y., Hagimoto N., Nakanishi Y., Hara N. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996 Aug;154(2 Pt 1):477–483. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- Larson E. W., Edwards W. D. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984 Mar 1;53(6):849–855. doi: 10.1016/0002-9149(84)90418-1. [DOI] [PubMed] [Google Scholar]
- Leri A., Claudio P. P., Li Q., Wang X., Reiss K., Wang S., Malhotra A., Kajstura J., Anversa P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest. 1998 Apr 1;101(7):1326–1342. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Candales A., Holmes D. R., Liao S., Scott M. J., Wickline S. A., Thompson R. W. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997 Mar;150(3):993–1007. [PMC free article] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Miyashita T., Harigai M., Hanada M., Reed J. C. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994 Jun 15;54(12):3131–3135. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995 Jan 27;80(2):293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Oren M. Relationship of p53 to the control of apoptotic cell death. Semin Cancer Biol. 1994 Jun;5(3):221–227. [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Rotter V., Aloni-Grinstein R., Schwartz D., Elkind N. B., Simons A., Wolkowicz R., Lavigne M., Beserman P., Kapon A., Goldfinger N. Does wild-type p53 play a role in normal cell differentiation? Semin Cancer Biol. 1994 Jun;5(3):229–236. [PubMed] [Google Scholar]
- Ruoslahti E., Reed J. C. Anchorage dependence, integrins, and apoptosis. Cell. 1994 May 20;77(4):477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Schlatmann T. J., Becker A. E. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977 Jan;39(1):13–20. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- Schlatmann T. J., Becker A. E. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977 Jan;39(1):21–26. doi: 10.1016/s0002-9149(77)80005-2. [DOI] [PubMed] [Google Scholar]
- Stary H. C., Chandler A. B., Glagov S., Guyton J. R., Insull W., Jr, Rosenfeld M. E., Schaffer S. A., Schwartz C. J., Wagner W. D., Wissler R. W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994 May;89(5):2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Teiger E., Than V. D., Richard L., Wisnewsky C., Tea B. S., Gaboury L., Tremblay J., Schwartz K., Hamet P. Apoptosis in pressure overload-induced heart hypertrophy in the rat. J Clin Invest. 1996 Jun 15;97(12):2891–2897. doi: 10.1172/JCI118747. [DOI] [PMC free article] [PubMed] [Google Scholar]