Abstract
OBJECTIVE—To determine preoperatively, by analysing asynchronous left ventricular wall motion, whether to approach through the right ventricle or the left ventricle when carrying out catheter ablation of the accessory pathway in Wolff-Parkinson-White syndrome, especially in patients with the pathway located on the septum. METHODS—73 patients with manifest Wolff-Parkinson-White syndrome who underwent successful catheter ablation were studied. Location of accessory pathway was classified as right ventricular side: right anterior paraseptum, right anterior, right lateral, right posterior, anterior septum, midseptum, right posterior septum; left ventricular side: left posterior septum, left posterior, left lateral, left anterior. Asynchronous systolic wall motion was analysed by cross sectional echocardiography. RESULTS—Echocardiography showed that the amplitude of left ventricular posterior systolic wall motion was reduced when the pathway was located on the left ventricular side as opposed to the right ventricular side (mean (SD), 11.1 (1.7) v 12.9 (1.1) mm, p < 0.001), especially in patients with left posterior septal accessory pathway (9.7 (0.8) mm). There were no overlapping values between the left posterior septal accessory pathway and the right ventricular side accessory pathway. Posterior wall notch motion was observed in all patients with a left posterior septal accessory pathway (9/9), but not at all in patients with pathways located on the right ventricular side of the septum. In patients with a septal accessory pathway, an ECG algorithm provided poor information (relatively low sensitivity, specificity, and predictive value) for determining whether the subsite faced either the left (left posterior septum) or the right ventricle (anterior septum, midseptum, right posterior septum). CONCLUSIONS—Decreased amplitude of left ventricular posterior wall motion with notch movement is an important finding for accessory pathways located on the left posterior septum. These findings provided clinically useful information for determining whether to approach catheter ablation from the right or the left ventricle. Keywords: echocardiography; catheter ablation; pre-excitation; asynchronous wall motion; Wolff-Parkinson-White syndrome
Full Text
The Full Text of this article is available as a PDF (220.5 KB).
Figure 1 .
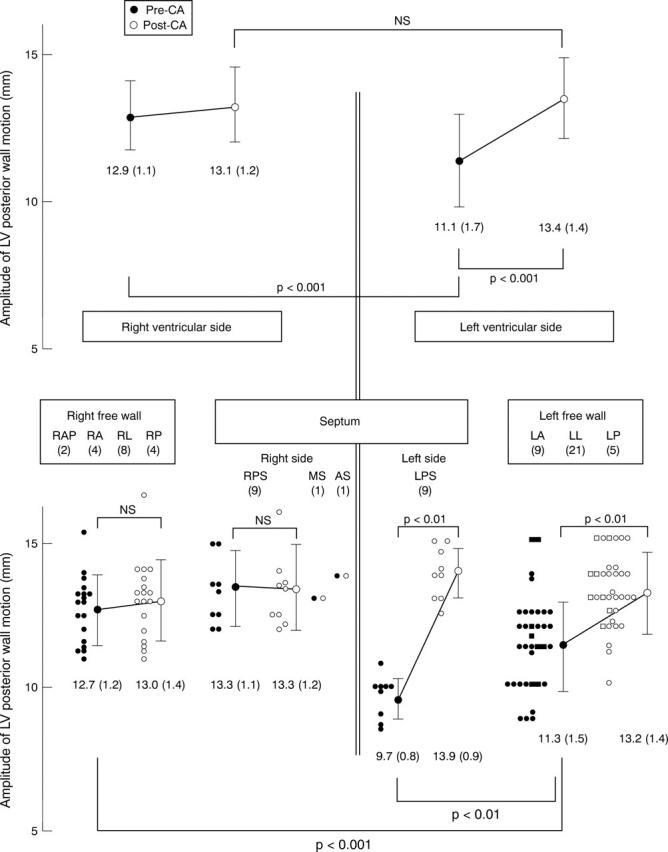
Amplitude of left ventricular posterior wall motion and accessory pathway location before and after successful catheter ablation. Empty and filled circles indicate the amplitude of posterior wall motion before and after catheter ablation, respectively, in patients with a QRS duration of ⩾ 0.12 s. Empty and filled squares indicate the amplitude of posterior wall motion before and after catheter ablation, respectively, in patients with a QRS duration of < 0.12 s. Numbers in parentheses indicate the numbers of patients. AS, anteroseptum; CA, catheter ablation; LA, left anterior free wall; LL, left lateral free wall; LP, left posterior free wall; LPS, left posterior septum; LV, left ventricle; MS, midseptum; RA, right anterior; RAP, right anterior paraseptum; RL, right lateral; RP, right posterior, RPS, right posterior septum.
Figure 2 .
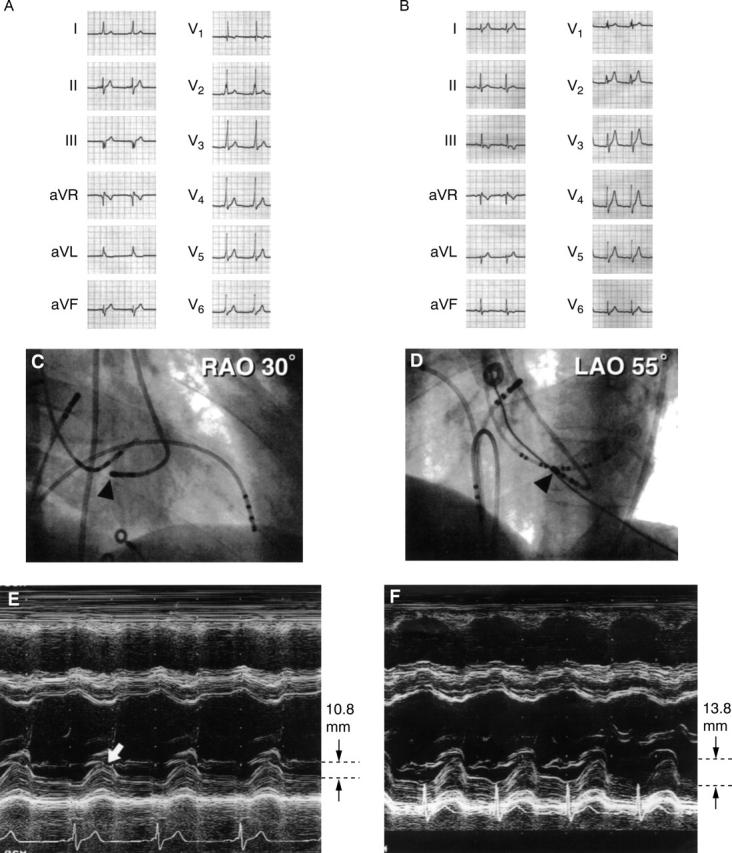
A patient with left posterior septal accessory pathway. (A) and (B), ECG recording before and after ablation. On the basis of the Oklahoma ECG algorithm, all three cardiologists assessed the location to be left posterior septum; on the basis of the St George's algorithm, the location was left posterior. (C) Right anterior view and (D) left anterior view: catheter position (indicated by arrowheads) for radiofrequency ablation. The catheter was positioned at the left posterior of the septum. (E) and (F): M mode echocardiography tracings before and after the ablation. Notch movement of the left ventricular posterior wall systolic motion can been seen in (E) (arrow) but the notch is not seen in (F). Reduced left ventricular posterior wall systolic wall motion (10.8 mm) can be seen before ablation. After ablation, the systolic motion was normalised (13.8 mm).
Figure 3 .
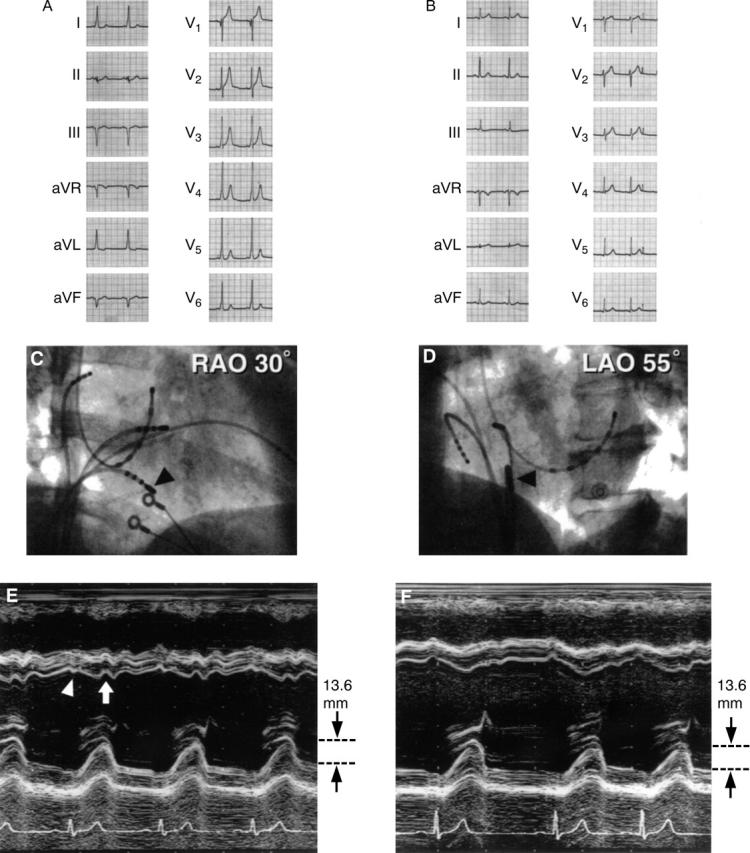
A patient with right posterior septal accessory pathway. (A) and (B): ECG recording before and after ablation. On the basis of the Oklahoma ECG algorithm, all three cardiologists assessed the location to be left posterior septum; on the basis of the St George's algorithm, one of the three assessed the location to be left posterior septum. (C) Right anterior view, and (D) left anterior view: catheter position (arrowheads) for radiofrequency ablation. Catheter was positioned at the right posterior septum. (E) and (F): M mode echocardiography tracings before and after ablation. Notch movement of the left ventricular posterior systolic motion cannot be seen in (D) and amplitude of left ventricular systolic motion was within the normal range. Pre-excitation wall motion—that is, early systolic movement (indicated by an arrowhead) and notch in the systolic septal motion (indicated by an arrow)—were observed in (E). Reduced posterior systolic wall motion cannot be seen. (F) After ablation, left ventricular systolic motion was unchanged.
Figure 4 .
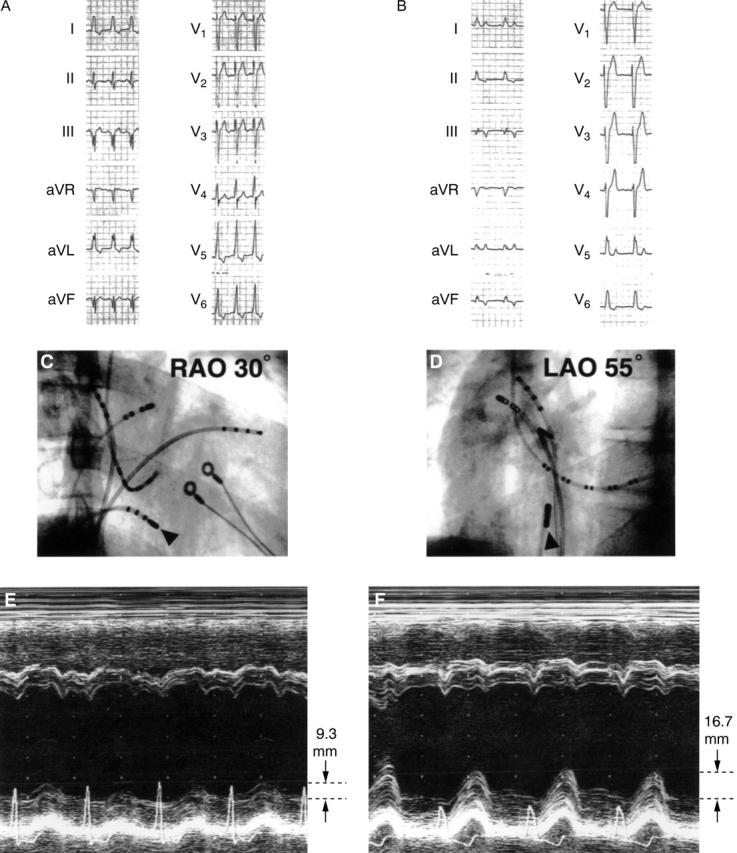
A patient with right posterior septal accessory pathway and complete left bundle branch block. (A) and (B): ECG recording before and after ablation. Left bundle branch block was observed after successful catheter ablation. (C) (right anterior view) and (D) (left anterior view): Catheter position (indicated by arrowheads) for radio frequency ablation. The catheter was positioned at the right posterior of the septum. (E) and (F): Tracings of M mode echocardiography before and after ablation. Paradoxical septal motion and markedly reduced left ventricular posterior wall systolic motion (9.3 mm) can be seen before ablation in (E). Improvement of left ventricular posterior wall systolic motion (16.7 mm) was observed after successful ablation in (F).
Figure 5 .
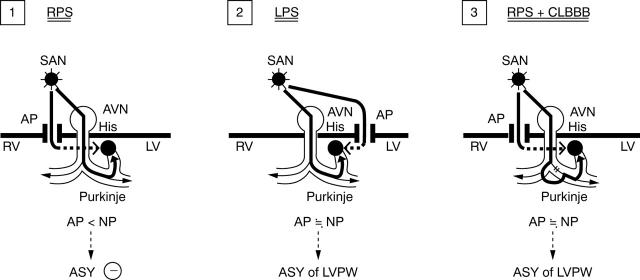
Speculation on the mechanism causing asynchronous left ventricular posterior wall systolic motion. (1) RPS, accessory pathway located in the right posterior septum. Electric current by the accessory pathway (AP) does not reach left ventricular wall during the systolic phase, resulting in the absence of asynchronous (ASY) left ventricular wall motion. (2) LPS, accessory pathway located in the left posterior septum. Electric current by the accessory pathway reaches the left ventricular wall in close timing with that by normal pathway (NP) during the systolic phase, resulting in asynchronous left ventricular wall systolic motion. (3) RPS + CLBBB, right posterior septum accessory pathway with complete left bundle branch block. Because of delayed conduction through the normal pathway owing to complete left bundle branch block, electric current by the right posterior septal pathway reaches the left ventricular wall in close timing with that by the normal pathway, resulting in asynchronous left ventricular wall motion. AVN, atrioventricular node; His, His bundle; LPS, left posterior septal accessory pathway; LV, left ventricle; LVPW, left ventricular posterior wall; RPS, right posterior septal accessory pathway; RV, right ventricle; SAN, sinoatrial node.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Ambroggi L., Taccardi B., Macchi E. Body-surface maps of heart potentials: tentative localization of pre-excited areas in forty-two Wolff-Parkinson-White patients. Circulation. 1976 Aug;54(2):251–263. doi: 10.1161/01.cir.54.2.251. [DOI] [PubMed] [Google Scholar]
- Dhala A. A., Deshpande S. S., Bremner S., Hempe S., Sra J. S., Blanck Z., Akhtar M., Jazayeri M. R. Transcatheter ablation of posteroseptal accessory pathways using a venous approach and radiofrequency energy. Circulation. 1994 Oct;90(4):1799–1810. doi: 10.1161/01.cir.90.4.1799. [DOI] [PubMed] [Google Scholar]
- Durrer D., van Dam R. T., Freud G. E., Janse M. J., Meijler F. L., Arzbaecher R. C. Total excitation of the isolated human heart. Circulation. 1970 Jun;41(6):899–912. doi: 10.1161/01.cir.41.6.899. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick A. P., Gonzales R. P., Lesh M. D., Modin G. W., Lee R. J., Scheinman M. M. New algorithm for the localization of accessory atrioventricular connections using a baseline electrocardiogram. J Am Coll Cardiol. 1994 Jan;23(1):107–116. doi: 10.1016/0735-1097(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Francis G. S., Theroux P., O'Rourke R. A., Hagan A. D., Johnson A. D. An echocardiographic study of interventricular septal motion in the Wolff-Parkinson-White syndrome. Circulation. 1976 Aug;54(2):174–178. doi: 10.1161/01.cir.54.2.174. [DOI] [PubMed] [Google Scholar]
- Gallagher J. J., Pritchett E. L., Sealy W. C., Kasell J., Wallace A. G. The preexcitation syndromes. Prog Cardiovasc Dis. 1978 Jan-Feb;20(4):285–327. doi: 10.1016/0033-0620(78)90015-4. [DOI] [PubMed] [Google Scholar]
- Jackman W. M., Wang X. Z., Friday K. J., Roman C. A., Moulton K. P., Beckman K. J., McClelland J. H., Twidale N., Hazlitt H. A., Prior M. I. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991 Jun 6;324(23):1605–1611. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- Kuck K. H., Schlüter M., Geiger M., Siebels J., Duckeck W. Radiofrequency current catheter ablation of accessory atrioventricular pathways. Lancet. 1991 Jun 29;337(8757):1557–1561. doi: 10.1016/0140-6736(91)93258-b. [DOI] [PubMed] [Google Scholar]
- Kuecherer H. F., Abbott J. A., Botvinick E. H., Scheinman E. D., O'Connell J. W., Scheinman M. M., Foster E., Schiller N. B. Two-dimensional echocardiographic phase analysis. Its potential for noninvasive localization of accessory pathways in patients with Wolff-Parkinson-White syndrome. Circulation. 1992 Jan;85(1):130–142. doi: 10.1161/01.cir.85.1.130. [DOI] [PubMed] [Google Scholar]
- Kuecherer H. F., Kleber Gda S., Melichercik J., Schützendübel R., Beyer T., Brachmann J., Kübler W. Transesophageal echo phase imaging for localizing accessory pathways during adenosine-induced preexcitation in patients with the Wolff-Parkinson-White syndrome. Am J Cardiol. 1996 Jan 1;77(1):64–71. doi: 10.1016/s0002-9149(97)89136-9. [DOI] [PubMed] [Google Scholar]
- Milstein S., Sharma A. D., Guiraudon G. M., Klein G. J. An algorithm for the electrocardiographic localization of accessory pathways in the Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. 1987 May;10(3 Pt 1):555–563. doi: 10.1111/j.1540-8159.1987.tb04520.x. [DOI] [PubMed] [Google Scholar]
- Rakovec P., Kranjec I., Fettich J. J., Fidler V., Pohar B., Porenta M., Janezic A., Varl B. Detection of electrocardiographically imperceptible ventricular pre-excitation by phase imaging. Clin Cardiol. 1986 Oct;9(10):475–478. doi: 10.1002/clc.4960091001. [DOI] [PubMed] [Google Scholar]
- Wen M. S., Yeh S. J., Wang C. C., King A., Lin F. C., Wu D. Radiofrequency ablation therapy of the posteroseptal accessory pathway. Am Heart J. 1996 Sep;132(3):612–620. doi: 10.1016/s0002-8703(96)90246-x. [DOI] [PubMed] [Google Scholar]
- Xie B., Heald S. C., Bashir Y., Katritsis D., Murgatroyd F. D., Camm A. J., Rowland E., Ward D. E. Localization of accessory pathways from the 12-lead electrocardiogram using a new algorithm. Am J Cardiol. 1994 Jul 15;74(2):161–165. doi: 10.1016/0002-9149(94)90090-6. [DOI] [PubMed] [Google Scholar]
- d'Avila A., Brugada J., Skeberis V., Andries E., Sosa E., Brugada P. A fast and reliable algorithm to localize accessory pathways based on the polarity of the QRS complex on the surface ECG during sinus rhythm. Pacing Clin Electrophysiol. 1995 Sep;18(9 Pt 1):1615–1627. doi: 10.1111/j.1540-8159.1995.tb06983.x. [DOI] [PubMed] [Google Scholar]