Abstract
Mice deficient in the hematopoietic cell-specific adapter protein SLP-76 demonstrate a failure of T cell development and fetal hemorrhage. Although SLP-76-deficient platelets manifest defective collagen receptor signaling, this alone may not explain the observed bleeding diathesis. Because αIIbβ3, the platelet fibrinogen receptor, is required for normal hemostasis, we explored a potential role for SLP-76 in αIIbβ3 signaling. Interaction of soluble or immobilized fibrinogen with normal human or murine platelets triggers rapid tyrosine phosphorylation of SLP-76. Moreover, platelet adhesion to fibrinogen stimulates actin rearrangements, filopodial and lamellipodial extension, and localization of tyrosine phosphorylated proteins to the cell periphery. In contrast, SLP-76-deficient murine platelets bind fibrinogen normally, but spread poorly and exhibit reduced levels of phosphotyrosine. The in vivo bleeding diathesis as well as the defects in platelet responses to fibrinogen and collagen are reversed by retroviral transduction of SLP-76 into bone marrow derived from SLP-76-deficient mice. These studies establish that SLP-76 functions downstream of αIIbβ3 and collagen receptors in platelets. Furthermore, expression of SLP-76 in hematopoietic cells, including platelets, plays a necessary role in hemostasis.
Recently, it has become evident that many molecules important for regulation of signaling events leading to lymphocyte activation also play critical roles in platelet function (1). One such molecule is SLP-76, a hematopoietic cell-specific adapter (2) that interacts with other signaling molecules through amino-terminal phosphotyrosine residues (3), a central proline-rich region, and a carboxyl-terminal Src homology 2 domain (SH2). Overexpression of SLP-76 in Jurkat T cells results in augmentation of signals initiated by the T cell antigen receptor (TCR) (4–6), whereas loss of SLP-76 uncouples the TCR from its signal transduction machinery (7). Additionally, mice made deficient for SLP-76 lack T cells (8, 9), likely due to the failure of the pre-TCR to signal during thymocyte development.
In addition to the T cell phenotype, SLP-76-deficient mice exhibit a bleeding diathesis and decreased perinatal survival (8, 9). Whereas megakaryocytes and platelets appear to develop normally in SLP-76−/− mice, SLP-76-deficient platelets fail to aggregate in response to collagen, although they respond to thrombin (10). An explanation for the role of SLP-76 in collagen-mediated signaling was provided by the observation that the collagen receptor, glycoprotein VI (GPVI), complexes with the common Fcγ chain (11, 12). Similar to the TCR and pre-TCR (13), Fcγ possesses an immunoreceptor tyrosine-based activation motif within its cytoplasmic tail (1). Upon collagen binding to GPVI, Fcγ is phosphorylated within the immunoreceptor tyrosine-based activation motif and recruits and activates the Syk protein tyrosine kinase (14). In SLP-76-deficient platelets, collagen binding still stimulates Syk, but key downstream substrates, e.g., phospholipase Cγ2, are not phosphorylated or activated (10, 15). However, it is unlikely that this defect in platelet function is sufficient to cause the severe bleeding phenotype observed in SLP-76−/− mice. For example, genetically altered mice deficient in other key components of the collagen-mediated signaling pathway do not exhibit a similar defect in hemostasis (16, 17). Moreover, platelets from mice deficient in the common Fcγ chain fail to aggregate in response to collagen; nonetheless, these mice do not manifest increased bleeding or decreased survival (17). In contrast, Syk-deficient mice have a lethal bleeding diathesis causing nearly all mice to die perinatally (18–20). Because Syk is known to play a role in several platelet activation pathways (21–25), it is possible that defects in one or more of these pathways, combined with the defect in signaling via GPVI, are responsible for the bleeding phenotype of SLP-76-null mice.
Ligation of αIIbβ, an integrin whose ligand-binding function is activated by inside-out signals, also stimulates Syk activation, leading to cytoskeletal rearrangements and platelet shape change (22, 26, 27). Because both the collagen receptor and αIIbβ3 use Syk, we asked whether SLP-76 participates in inside-out and/or outside-in signaling through αIIbβ3. In these studies, we show that SLP-76 becomes tyrosine phosphorylated after fibrinogen binding to αIIbβ3 in normal human and murine platelets. Consistent with a functional role for SLP-76 in outside-in signaling through αIIbβ3, platelets from SLP-76-deficient mice exhibit reduced tyrosine phosphorylation of cellular substrates in response to fibrinogen. Additionally, SLP-76-null platelets spread poorly on a fibrinogen matrix, suggesting that this adapter also is required for optimal signaling from αIIbβ3 to the actin cytoskeleton. Finally, through retroviral gene transfer into SLP-76-deficient bone marrow, we demonstrate that reconstitution of SLP-76 in murine platelets reverses both the bleeding diathesis and the defects in platelet signaling observed in the mutant mice.
Materials and Methods
Antibodies, Reagents, and Mice.
Ligand-induced binding site (LIBS)-6 Fab, Ro43–5054, and convulxin were gifts of M. Ginsberg (The Scripps Research Institute, La Jolla, CA), B. Steiner (Roche, Basel, Switzerland), and J. Gibbons (The University of Reading, Reading, U.K.), respectively. Anti-P-selectin was from PharMingen. Purified human fibrinogen and α-thrombin were from Enzyme Research Laboratories (South Bend, IN), collagen was from Chronolog (Havertown, PA), and Cytochalasin D was from Sigma. FITC-anti-mouse IgG was from BioSource International (Camarillo, CA), Cy5-anti-mouse IgG was from Jackson ImmunoResearch, and rhodamine-phalloidin was from Molecular Probes. Recombinant hematopoietic growth factors were from R & D Systems. SLP-76-deficient mice were housed under pathogen-free conditions at the University of Iowa or University of Pennsylvania Animal Care Facilities under National Institutes of Health guidelines and approved animal protocols. Wild-type C57/BL6 and RAG2−/− (The Jackson Laboratory) mice were used as indicated.
Platelet Isolation.
Whole blood was obtained from 4- to 8-wk-old mice by cardiac puncture and anticoagulated with 0.1 vol acidic-citrate-dextrose. Four milliliters of Pipes-saline buffer (150 mM NaCl/20 mM Pipes, pH 6.5) was added, and platelet-rich plasma was obtained by centrifugation at 200 × g for 20 min at 25°C. When stated, 1 mM aspirin was added at this step for 20 min at 37°C to irreversibly inhibit platelet cyclooxygenase. Washed platelets then were obtained by adding apyrase (10 units/ml final concentration) and prostaglandin E1 (1 μM), centrifuging at 600 × g for 20 min at 25°C, and resuspending the platelet pellet in a modified Tyrode's buffer (134 mM NaCl/2.9 mM KCl/0.34 mM Na2HPO4-12H2O/12 mM NaHCO3/20 mM Hepes/1 mM MgCl2/5 mM glucose, pH 7.35). Human whole blood was obtained from medication-free volunteers and washed platelets were prepared as described (28).
Immunoprecipitation and Western Blotting.
Bacterial Petri dishes or glass coverslips were coated overnight at 4°C with 100 μg/ml fibrinogen or 5 mg/ml BSA in coating buffer (150 mM NaCl/50 mM NaH2PO4/50 mM Na2HPO4, pH 8.0), followed by blocking in 5 mg/ml BSA for 2 h at room temperature. Platelet adhesion to fibrinogen was carried out by adding washed platelets (1 × 108 platelets/ml) to the dishes or coverslips for 45 min at 37°C. After removing nonadherent platelets from the fibrinogen-coated Petri dishes with three washes of PBS, adherent platelets were lysed for 30 min on ice in 1% Nonidet P-40 lysis buffer (1% Nonidet P-40/150 mM NaCl/50 mM Tris, pH 7.4) including inhibitors of proteases (50 μg/ml aprotinin/10 μg/ml leupeptin/50 μg/ml pepstatin A/1 mM phenylmethylsulfonyl fluoride) and phosphatases (400 μM sodium vanadate/10 mM sodium fluoride/10 mM sodium pyrophosphate). Platelets incubated in BSA-coated dishes served as suspension controls and were pelleted for 5 sec at 16,000 × g before lysis. Clarified lysates were subjected to immunoprecipitation with sheep anti-murine SLP-76 (4), and immunoprecipitates were probed on Western blots with antiphosphotyrosine mAbs, 4G10 (Upstate Biotechnology, Lake Placid, NY) and PY20 (Transduction Laboratories, Lexington, KY), and immunoreactive bands were detected by enhanced chemiluminescence (ECL; Amersham Pharmacia).
Confocal Microscopy and Scanning Electron Microscopy (SEM).
Washed platelets were allowed to adhere to fibrinogen-coated coverslips for 45 min at 37°C. Coverslips were then washed and platelets were fixed in 1% gluteraldehyde for SEM or PermeaFix (Ortho Diagnostic Systems, Raritan, NJ) for confocal microscopy. Coverslips for SEM were dehydrated and sputter-coated with gold followed by analysis using an Hitachi S-4000 (University of Iowa Microscopy Facility). Coverslips for confocal microscopy were stained with 4G10 and PY20, FITC-anti-mouse IgG or Cy5-anti-mouse IgG, and rhodamine-phalloidin, and confocal microscopy was carried out as described (28).
Flow Cytometry.
Washed murine platelets (1 × 106) were stimulated with 0.5 unit/ml human α-thrombin or 50 ng/ml convulxin in the presence of either 200 μg/ml FITC-fibrinogen or 5 mg/ml biotinylated anti-murine P-selectin, and fibrinogen binding was quantified by flow cytometry (29).
Genetic Reconstitution of SLP-76-Deficient Bone Marrow.
A 1.6-kb murine SLP-76 cDNA was cloned into the BglII site of the MigR1 vector (30). This BglII site lies directly 5′ of an internal ribosome entry site, which is followed by a cDNA coding for enhanced green fluorescent protein (GFP). Bosc23 cells were transfected with MIGR1 and viral supernatants were collected as described (31).
Bone marrow cells were harvested from the tibias and femurs of SLP-76-deficient mice and cultured for 4 days at a density of 2 × 106 cells/ml in the presence of 6 ng/ml rmIL-3, 10 ng/ml rmIL-6, and 50 ng/ml rmSCF (stem cell factor) in DMEM containing 15% FCS and 5% WEHI-conditioned medium. At 24 and 48 h, cells were spin infected with retroviral supernatants followed by i.v. injection into lethally irradiated 4- to 6-week-old C57/BL6 or RAG2−/− recipients as described (30).
Results
SLP-76 Is Tyrosine Phosphorylated in Human Platelets After Fibrinogen Binding to αIIbβ3.
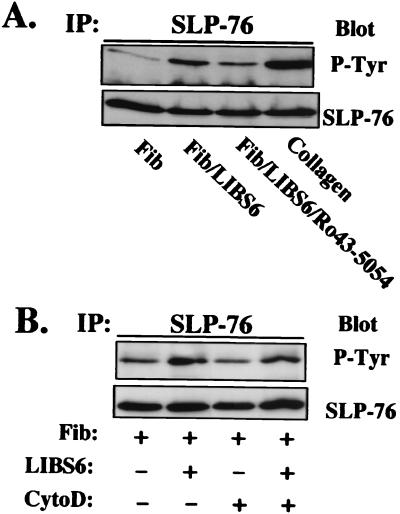
We have shown previously that SLP-76 is tyrosine phosphorylated in platelets after engagement of GPVI (10, 15), a collagen receptor that is coupled to Syk. Because Syk also is activated by outside-in signals mediated by αIIbβ3 (22), we asked whether binding of soluble fibrinogen to αIIbβ3 results in SLP-76 tyrosine phosphorylation. Washed human platelets were incubated with Fab fragments of LIBS-6, an antibody specific for β3 that directly converts αIIbβ3 to a high-affinity fibrinogen receptor. Fibrinogen binding induced by LIBS-6 Fab is known to result in tyrosine phosphorylation of multiple platelet substrates (32). The experiment shown in Fig. 1A demonstrates that SLP-76 is one of these substrates [compare lane 1 (fibrinogen alone) with lane 2 (fibrinogen + LIBS-6 Fab) or lane 4 (collagen)]. Tyrosine phosphorylation of SLP-76 induced by LIBS-6 Fab plus fibrinogen is effectively blocked by Ro43–5054, a selective αIIbβ3 antagonist (lane 3). Thus, fibrinogen binding to αIIbβ3 triggers tyrosine phosphorylation of SLP-76 in human platelets.
Figure 1.
SLP-76 is tyrosine phosphorylated in response to fibrinogen binding to αIIbβ3 independent of actin polymerization. (A) Platelets were incubated with 250 μg/ml human fibrinogen (lane 1), fibrinogen plus 150 μg/ml LIBS-6 Fab (lane 2), fibrinogen plus LIBS-6 Fab plus 10 μM Ro43–5054 (lane 3), or 5 μg/ml collagen (lane 4). Platelets were lysed, immunoprecipitated with anti-human SLP-76, and analyzed by SDS/PAGE and immunoblot for phosphotyrosine. The top blot was stripped and reprobed with anti-human SLP-76 to demonstrate equal amounts of immunoprecipitated SLP-76 in each lane. Similar results were obtained in three separate experiments. (B) Washed human platelets were incubated for 10 min at room temperature in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of 10 μM cytochalasin D and then stimulated with 250 μg/ml fibrinogen alone (lanes 1 and 3), or 150 μg/ml LIBS-6 Fab plus 250 μg/ml fibrinogen (lanes 2 and 4).
Once fibrinogen binds to αIIbβ3, platelets undergo aggregation and spreading (33–35), responses associated with actin polymerization and reorganization. Some signaling reactions triggered by fibrinogen binding, such as activation of focal adhesion kinase (FAK), depend on actin polymerization (36), whereas others, including tyrosine phosphorylation of Syk and Vav, are not (37). To determine whether tyrosine phosphorylation of SLP-76 depends on actin polymerization, platelets were preincubated for 10 min with 10 μM cytochalasin D, an inhibitor of actin polymerization. Under these conditions, LIBS-6 Fab plus fibrinogen still induces tyrosine phosphorylation of SLP-76 (Fig. 1B). Thus, integrin-dependent tyrosine phosphorylation of SLP-76 is independent of actin polymerization, similar to what is seen for Syk and Vav.
SLP-76 Is Tyrosine Phosphorylated After Engagement of αIIbβ3 in Murine Platelets.
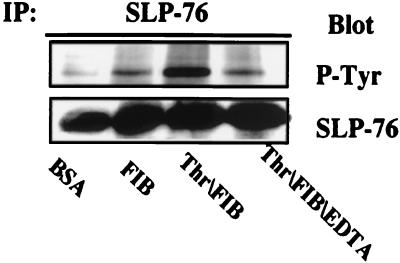
To determine whether SLP-76 is a substrate of the protein tyrosine kinases stimulated by ligation of αIIbβ3 in murine platelets, we examined the status of SLP-76 tyrosine phosphorylation in response to adhesion of murine platelets to immobilized fibrinogen, an interaction that can occur in the absence of prior platelet activation (38) (Fig. 2). The platelets were pretreated with aspirin and then incubated on culture dishes coated with fibrinogen or BSA. Platelets did not adhere to the BSA-coated dishes, nor did these cells exhibit tyrosine phosphorylation of SLP-76 (lane 1). In contrast, platelets adherent to fibrinogen manifest SLP-76 phosphorylation (lanes 2 and 3). Both the adhesion and phosphorylation are blocked by addition of 10 mM EDTA (lane 4), a treatment known to prevent the interaction of fibrinogen with the activated integrin. These data demonstrate that tyrosine phosphorylation of SLP-76 can be triggered in murine platelets, as in human platelets, by the interaction of fibrinogen with αIIbβ3.
Figure 2.
Stimulation of murine platelets on a fibrinogen-coated surface induces αIIbβ3-dependent tyrosine phosphorylation of SLP-76. Washed murine platelets were incubated for 45 min at 37°C on Petri dishes coated with 5 mg/ml BSA (lane 1) or 100 μg/ml human fibrinogen (lanes 2–4) in the absence (lanes 1 and 2) or presence of 1 unit/ml thrombin (lane 3) or 1 unit/ml thrombin plus 10 mM EDTA to block fibrinogen binding to αIIbβ3 (lane 4). Nonadherent platelets were isolated by centrifugation (5 sec, 16,000 × g) and then lysed (lanes 1 and 4). Fibrinogen-adherent platelets were lysed directly on the Petri dishes (lanes 2 and 3). Equal amounts of protein from each lysate were subjected to immunoprecipitation with anti-murine SLP-76 followed by Western analysis for phosphotyrosine. Blots were reprobed with anti-murine SLP-76 to demonstrate equal amounts of immunoprecipitated SLP-76 in each lane.
Inside-Out Signaling to αIIbβ3 Is Intact in SLP-76-Deficient Platelets.
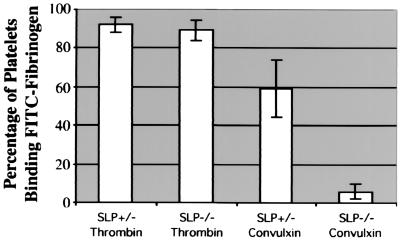
Treatment of platelets with thrombin leads to activation of and fibrinogen binding to αIIbβ3 (27). Because of the hemostatic defects in SLP-76-deficient mice, we investigated whether their platelets are capable of transmitting inside-out signals to αIIbβ3 and binding fibrinogen after thrombin stimulation. As expected, engagement of GPVI with the snake venom agonist, convulxin, stimulated increased FITC-fibrinogen binding to SLP-76+/− platelets, but not to SLP-76−/− cells (Fig. 3). In contrast, activation of platelets with 0.5 unit/ml thrombin stimulated increased FITC-fibrinogen binding to both SLP-76+/− and SLP-76−/− platelets. These results indicate that there is no global defect in inside-out signaling to αIIbβ3 in SLP-76-deficient platelets.
Figure 3.
Thrombin stimulation of platelets from SLP-76-deficient and heterozygous mice results in comparable activation of αIIbβ3. Washed murine platelets were left unstimulated or stimulated with 0.5 unit/ml thrombin or 50 ng/ml convulxin for 10 min at 37°C. Cells then were incubated with 200 μg/ml FITC-fibrinogen and analyzed by flow cytometry, normalizing for the unstimulated controls. Results from three separate experiments are shown with error bars representing SD of the mean.
Deficiency of SLP-76 Impairs αIIbβ3-Dependent Tyrosine Phosphorylation and Shape Change in Platelets.
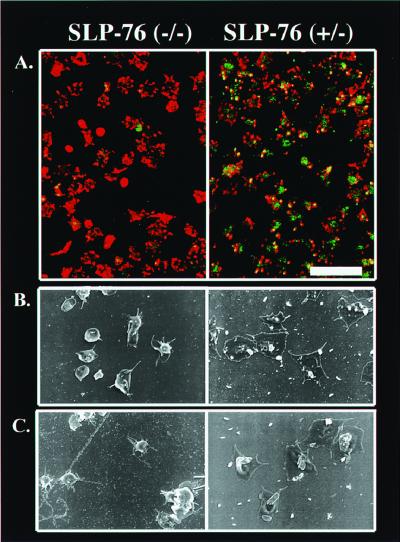
To assess the potential functional consequences of SLP-76 deficiency on events downstream of αIIbβ3 engagement, we compared fibrinogen-induced tyrosine phosphorylation of cellular substrates and shape change in platelets obtained from SLP-76 heterozygous and SLP-76-deficient mice. After adherence to fibrinogen-coated coverslips, platelets were fixed, permeabilized, and stained with anti-phosphotyrosine antibodies and FITC-conjugated secondary antibody to assess global tyrosine phosphorylation, and with rhodamine-phalloidin to visualize F-actin. As shown in the composite confocal images in Fig. 4A, heterozygous platelets exhibit numerous filopodia and in some cases full spreading, and phosphotyrosine is prominent throughout the cell, including at the periphery (Right). In contrast, although SLP-76-deficient platelets adhere to fibrinogen, they exhibit a substantial reduction in spreading and tyrosine phosphorylation (Left). A similar reduction in global tyrosine phosphorylation of cellular substrates was obtained by Western analysis of whole cell lysates immunoblotted with anti-phosphotyrosine antibodies (data not shown).
Figure 4.
Deficiency of SLP-76 impairs αIIbβ3-dependent tyrosine phosphorylation and shape change in platelets. (A) Washed SLP-76−/− or SLP-76+/− platelets were incubated on coverslips coated with 100 μg/ml fibrinogen. Cells were permeabilized, fixed, and then stained for phosphotyrosine (green) and F-actin (red). The bar in the lower right corner corresponds to 10 μm. (B and C) Washed SLP-76−/− or SLP-76+/− platelets were placed on fibrinogen-coated coverslips and left unstimulated (B) or stimulated with 30 μg/ml collagen (C). Cells were then examined by SEM. Each image is ×5,000. Results shown are typical of three separate experiments.
To evaluate platelet spreading further, fibrinogen-adherent platelets also were examined by SEM. As shown in Fig. 4, this technique confirms the dramatic spreading defect in SLP-76−/− platelets. Collectively, these results indicate that the defect in αIIbβ3-dependent tyrosine phosphorylation in SLP-76-deficient platelets correlates with a defect in cytoskeletal reorganization and cell spreading. Additionally, consistent with our previous finding of a collagen receptor signaling defect in SLP-76-deficient platelets (10), SEM analysis confirms a spreading defect on immobilized fibrinogen in SLP-76-null platelets stimulated with collagen (Fig. 4C).
In Vivo Reconstitution of SLP-76 Rescues the Bleeding Phenotype of SLP-76-Deficient Mice.
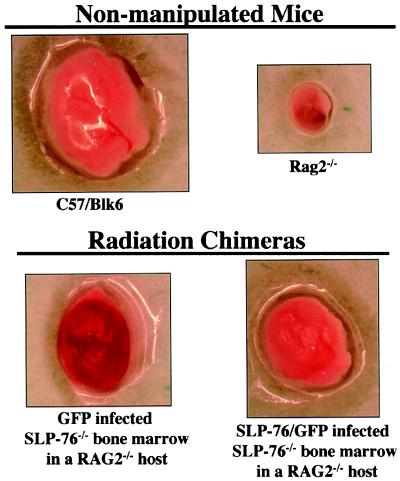
We and others have demonstrated that in addition to the signaling defects apparent in ex vivo studies of platelets from SLP-76-deficient mice, these animals demonstrate an in vivo-bleeding diathesis (8–10, 15). Although SLP-76 appears to be hematopoietic-cell specific (39), it is formally possible that defects in platelet function are not causal for the bleeding disorder in the SLP-76-null mice. To address this issue, lethally irradiated mice were reconstituted with bone marrow derived from SLP-76-deficient mice that had been infected with a control retrovirus expressing GFP or with a retrovirus encoding both SLP-76 and GFP. In some experiments wild-type (C57/BL6) mice were used as hosts and in other experiments the host animals were RAG2−/− mice. Lack of T and B lymphocytes and lymph nodes in nonmanipulated RAG2−/− mice permitted simple detection of successful reconstitution of the hematopoietic system by donor cells. However, results were similar regardless of the host strain used.
Approximately 6 wk after bone marrow reconstitution, mice were killed and tissues were harvested for study. Flow cytometric analysis of platelets from the reconstituted mice indicated that between 5% and 10% of the platelets were GFP positive, thus documenting successful infection of precursor cells in the bone marrow preparations (data not shown). As depicted in Fig. 5, gross examination of nonmanipulated C57/BL6 mice reveals normal-sized lymph nodes with no evidence of hemorrhage. Although lymph nodes from RAG2−/− mice are much smaller than those of normal mice, there is no evidence of bleeding within these nodes. However, when RAG2−/− hosts are reconstituted with SLP-76-deficient cells infected with the control virus (GFP), the lymph nodes become populated with B cells (data not shown) but also exhibit obvious hemorrhage. In marked contrast, reconstitution of RAG2−/− recipients with bone marrow infected with a SLP-76 expressing virus (Fig. 5, SLP-76/GFP) results in a similar nodal development but no evidence of hemorrhage. These results indicate that restoration of SLP-76 expression in the hematopoietic compartment reverses the bleeding diathesis characteristic of SLP-76-deficient mice. Furthermore, SLP-76 expression in only a small proportion of the platelets permits full protection from lymph node hemorrhage.
Figure 5.
Gene transfer of SLP-76 into SLP-76-deficient bone marrow restores hemostasis. Bone marrow harvested from SLP-76-deficient mice was infected with a retrovirus encoding GFP alone or GFP plus SLP-76 (SLP-76/GFP) and then administered i.v. into lethally irradiated Rag2−/− hosts. Mice were killed and tissues were harvested for study 6 wk later. Axillary lymph nodes were removed and photographed. Control lymph nodes from C57/BL6 and Rag2−/− mice are also shown. Similar results were seen in three independent experiments.
In Vivo Reconstitution of SLP-76 Restores Platelet Function in SLP-76−/− Mice.
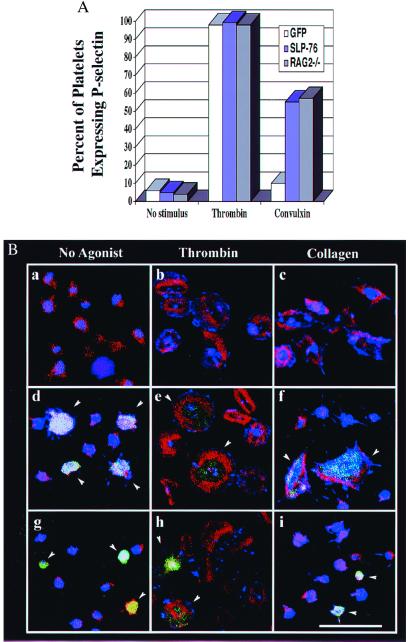
In addition to this gross measure of platelet function, we found that signal transduction via the collagen and fibrinogen receptors is restored in platelets obtained from the SLP-76 reconstituted mice. Only a fraction of platelets in each bone marrow host arise from retrovirally transduced precursors. However, because these platelets are marked with GFP, their function can be analyzed by flow cytometry and confocal microscopy. Washed platelets were left untreated or stimulated with thrombin or convulxin for 10 min. Cells were then assessed for expression of the P-selectin activation marker by flow cytometry. Gates were set to distinguish GFP-positive (transduced) vs. GFP-negative (nontransduced) cells. As shown in Fig. 6A, platelets from the control RAG2−/− mice undergo α-granule secretion, as monitored by surface P-selectin expression, in response to either convulxin or thrombin. In contrast, GFP-positive (and negative) platelets from irradiated mice given SLP-76-deficient bone marrow infected with GFP alone respond to thrombin but not to convulxin. However, the convulxin response of GFP-positive platelets is restored by reconstitution of irradiated mice with SLP-76-infected bone marrow. As expected, GFP-negative platelets from these same mice fail to respond to convulxin (data not shown). These results are comparable to those obtained in platelets from nonmanipulated SLP-76-deficient mice, which fail to express P-selectin on their surface in response to convulxin, whereas heterozygous littermate controls respond normally (data not shown).
Figure 6.
In vivo reconstitution of SLP-76 restores platelet function in SLP-76−/− mice. (A) Platelets were isolated from the nonmanipulated RAG2−/− mice or retrovirally reconstituted mice and left either untreated or stimulated with thrombin or convulxin for 10 min and then analyzed by flow cytometry for P-selectin expression. For the bone marrow reconstituted mice, gates were set to analyze only GFP-positive (transduced) cells. Similar results were seen in three independent experiments. (B) Platelets from nonmanipulated RAG2−/− mice or mice reconstituted with manipulated bone marrow were incubated for 45 min on fibrinogen-coated coverslips in the absence (a, d, and g) or presence of 0.5 unit/ml thrombin (b, e, and h), or 30 μg/ml collagen (c, f, and i). Cells were then fixed, permeabilized, and stained for phosphotyrosine (blue) and F-actin (red). Each field with platelets from manipulated mice contains a mixture of GFP-positive (transduced) and GFP-negative (nontransduced) platelets. Arrows indicate GFP-positive cells. Platelets from nonmanipulated RAG2−/− mice are shown in a-c, and d-f show platelets from a RAG2−/− mouse reconstituted with SLP-76−/− bone marrow expressing SLP-76 and GFP. (g–i) Platelets from a RAG2−/− mouse reconstituted with SLP-76−/− bone marrow expressing GFP. All panels are the same magnification. (Bar = 10 μm.)
To determine whether the spreading defect exhibited by SLP-76-deficient platelets is restored by reconstitution of SLP-76, fibrinogen-adherent platelets were evaluated by confocal microscopy. Platelets from nonmanipulated RAG2−/− mice spread on fibrinogen (Fig. 6Ba), and this response is enhanced in the presence of thrombin (Fig. 6Bb) or collagen (Fig. 6Bc). As expected, both GFP-positive and GFP-negative platelets from mice reconstituted with SLP-76-deficient bone marrow transduced with the virus encoding GPF alone spread poorly on fibrinogen, and spreading was enhanced by thrombin but not collagen (Fig. 6B g-i). In contrast, GFP-positive platelets from mice reconstituted with bone marrow transduced with virus encoding both GFP and SLP-76 spread normally on fibrinogen and respond to either thrombin or collagen (Fig. 6 Be and Bf). As shown also in these images, platelets derived from SLP-76-deficient bone marrow that were not successfully transduced (GFP-negative) still fail to spread after collagen stimulation, but maintain their response to thrombin. Collectively, these studies with reconstituted mice establish that restoration of SLP-76 expression rescues the in vivo-bleeding diathesis as well as the ex vivo platelet signaling defects characteristic of SLP-76 deficiency.
Discussion
In addition to T cell defects, mice deficient in the SLP-76 adapter protein manifest fetal hemorrhage and increased early mortality (8–10). Recent work has demonstrated that, similar to thymocytes, platelets from SLP-76-null mice demonstrate defects in signaling via cell surface receptors by using immunoreceptor tyrosine-based activation motifs (e.g., the pre-TCR in thymocytes and GPVI in platelets) (10, 15). However, work with other strains of genetically altered mice (such as those deficient in Fcγ) suggests strongly that impaired signaling via GPVI alone cannot result in the bleeding diathesis observed in the SLP-76-deficient mice (16, 17). In this report, we extend our studies of platelets from SLP-76-null mice, documenting defective signaling mediated by αIIbβ3 after engagement with fibrinogen. We demonstrate further, by using a retroviral-based gene transfer technique to reconstitute SLP-76 expression, that the in vivo hemostatic defect and the abnormalities in ex vivo platelet signal transduction are due to SLP-76 deficiency within the hematopoietic compartment.
Although it appears that SLP-76 plays a critical role in both GPVI and αIIbβ3 signaling, it still is unknown whether defects in signaling via these receptors alone explain the bleeding diathesis. Some insight into this question can be gained from studies of other mice with defined genetic abnormalities. In contrast to mice that lack Fcγ, mice deficient for the β3 integrin exhibit a bleeding phenotype with some similarities to that of SLP-76-deficient mice. However, β3−/− mice have prolonged bleeding times and their platelets cannot bind fibrinogen, abnormalities not seen in the SLP-76-deficient mice (40). More recently, mice have been described with engineered mutations in two tyrosine residues within the cytoplasmic tail of β3. Platelets from these mice still bind fibrinogen, but αIIbβ3-dependent clot retraction is reduced, and the mice have a predilection toward rebleeding after initial hemostasis (41). Of note, αIIbβ3 signaling also appears to be important for human platelet function as individuals with variant Glanzmann thrombasthenia have platelets which exhibit mutations in the β3 cytoplasmic tail and defects in bidirectional integrin signaling (42, 43). These observations suggest that signaling via αIIbβ3 is important for normal hemostasis in humans and mice. Taken together with our findings that SLP-76 is inducibly phosphorylated after αIIbβ3 engagement and a deficiency of SLP-76 is associated with impaired platelet spreading on fibrinogen, we conclude that SLP-76 functions as an integrator of signals downstream of αIIbβ3. The mechanism by which SLP-76 affects signaling via αIIbβ3 likely involves the molecules with which it is known to associate. In activated T lymphocytes SLP-76 binds several proteins known to influence actin polymerization and cytoskeletal organization, including Vav, Nck, and SLAP-130 (45, 46). Each of these proteins is present in platelets. Indeed, Vav has been implicated in promoting lamellipodia formation downstream of αIIbβ3 and Syk (37, 47).
In addition to further characterization of the effect of SLP-76 deficiency on signaling via GPVI and αIIbβ3, it will be important to examine the integrity of other signaling pathways in SLP-76-deficient platelets. Although platelet activation via thrombin receptors appears to be intact, ongoing studies are examining this and other agonist-induced signaling pathways in detail. Recent studies indicate that there is cross talk between receptors that activate protein tyrosine kinases and those that couple to heterotrimeric GTP-binding proteins (44), raising the possibility that SLP-76 may indirectly modulate signaling initiated by many platelet agonists.
Acknowledgments
We are grateful to M. Kahn and E. Peterson for helpful discussion and J. Clements and D. Adelman for technical assistance. This work was supported in part by grants from the National Institutes of Health (to G.K., S.S., and W.P.). W.P. is a recipient of a Scholar Award from the Leukemia and Lymphoma Society.
Abbreviations
- TCR
T cell receptor
- SEM
scanning electron microscopy
- GFP
green fluorescent protein
- LIBS
ligand-induced binding site
- GPVI
glycoprotein VI
References
- 1.Watson S P, Gibbins J. Immunol Today. 1998;19:260–264. doi: 10.1016/s0167-5699(98)01267-5. [DOI] [PubMed] [Google Scholar]
- 2.Jackman J K, Motto D G, Sun Q, Tanemoto M, Turck C W, Peltz G A, Koretzky G A, Findell P R. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 3.Myung P S, Boerthe N J, Koretzky G A. Curr Opin Immunol. 2000;12:256–266. doi: 10.1016/s0952-7915(00)00085-6. [DOI] [PubMed] [Google Scholar]
- 4.Motto D G, Ross S E, Wu J, Hendricks-Taylor L R, Koretzky G A. J Exp Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang N, Motto D G, Ross S E, Koretzky G A. J Immunol. 1996;157:3769–3773. [PubMed] [Google Scholar]
- 6.Wardenburg J B, Fu C, Jackman J K, Flotow H, Wilkinson S E, Williams D H, Johnson R, Kong G, Chan A C, Findell P R. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 7.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 8.Clements J L, Yang B, Ross-Barta S E, Eliason S L, Hrstka R F, Williamson R A, Koretzky G A. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 9.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt F W, Geha R S. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 10.Clements J L, Lee J R, Gross B, Yang B, Olson J D, Sandra A, Watson S P, Lentz S R, Koretzky G A. J Clin Invest. 1999;103:19–25. doi: 10.1172/JCI5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuji M, Ezumi Y, Arai M, Takayama H. J Biol Chem. 1997;272:23528–23531. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- 12.Gibbins J M, Okuma M, Farndale R, Barnes M, Watson S P. FEBS Lett. 1997;413:255–259. doi: 10.1016/s0014-5793(97)00926-5. [DOI] [PubMed] [Google Scholar]
- 13.Weiss A, Littman D R. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 14.Gibbins J, Asselin J, Farndale R, Barnes M, Law C L, Watson S P. J Biol Chem. 1996;271:18095–18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 15.Gross B S, Lee J R, Clements J L, Turner M, Tybulewicz V L, Findell P R, Koretzky G A, Watson S P. J Biol Chem. 1999;274:5963–5971. doi: 10.1074/jbc.274.9.5963. [DOI] [PubMed] [Google Scholar]
- 16.Pasquet J M, Gross B, Quek L, Asazuma N, Zhang W, Sommers C L, Schweighoffer E, Tybulewicz V, Judd B, Lee J R, et al. Mol Cell Biol. 1999;19:8326–8334. doi: 10.1128/mcb.19.12.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole A, Gibbins J M, Turner M, van Vugt M J, van de Winkel J G, Saito T, Tybulewicz V L, Watson S P. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng A M, Rowley B, Pao W, Hayday A, Bolen J B, Pawson T. Nature (London) 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 19.Turner M, Mee P J, Costello P S, Williams O, Price A A, Duddy L P, Furlong M T, Geahlen R L, Tybulewicz V L. Nature (London) 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 20.Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T. Mol Cell Biol. 1998;18:4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue K, Ozaki Y, Satoh K, Wu Y, Yatomi Y, Shin Y, Morita T. Biochem Biophys Res Commun. 1999;256:114–120. doi: 10.1006/bbrc.1999.0295. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Zoller K E, Ginsberg M H, Brugge J S, Shattil S J. EMBO J. 1997;16:6414–6425. doi: 10.1093/emboj/16.21.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallet C, Rosa J P, Habib A, Lebret M, Levy-Toledano S, Maclouf J. J Biol Chem. 1999;274:23610–23616. doi: 10.1074/jbc.274.33.23610. [DOI] [PubMed] [Google Scholar]
- 24.Pain S, Falet H, Saci A, Bachelot-Loza C, Rendu F. Cell Signal. 2000;12:165–171. doi: 10.1016/s0898-6568(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 25.Maschberger P, Bauer M, Baumann-Siemons J, Zangl K J, Negrescu E V, Reininger A J, Siess W. J Biol Chem. 2000;275:19159–19166. doi: 10.1074/jbc.M910257199. [DOI] [PubMed] [Google Scholar]
- 26.O'Toole T E, Mandelman D, Forsyth J, Shattil S J, Plow E F, Ginsberg M H. Science. 1991;254:845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- 27.Shattil S J. Thromb Haemostasis. 1999;82:318–325. [PubMed] [Google Scholar]
- 28.Leng L, Kashiwagi H, Ren X D, Shattil S J. Blood. 1998;91:4206–4215. [PubMed] [Google Scholar]
- 29.Law D A, Nannizzi-Alaimo L, Ministri K, Hughes P E, Forsyth J, Turner M, Shattil S J, Ginsberg M H, Tybulewicz V L, Phillips D R. Blood. 1999;93:2645–2652. [PubMed] [Google Scholar]
- 30.Pear W S, Miller J P, Xu L, Pui J C, Soffer B, Quackenbush R C, Pendergast A M, Bronson R, Aster J C, Scott M L, Baltimore D. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 31.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang M M, Lipfert L, Cunningham M, Brugge J S, Ginsberg M H, Shattil S J. J Cell Biol. 1993;122:473–483. doi: 10.1083/jcb.122.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haimovich B, Lipfert L, Brugge J S, Shattil S J. J Biol Chem. 1993;268:15868–15877. [PubMed] [Google Scholar]
- 34.Leong L, Hughes P E, Schwartz M A, Ginsberg M H, Shattil S J. J Cell Sci. 1995;108:3817–3825. doi: 10.1242/jcs.108.12.3817. [DOI] [PubMed] [Google Scholar]
- 35.Pelletier A J, Kunicki T, Ruggeri Z M, Quaranta V. J Biol Chem. 1995;270:18133–18140. doi: 10.1074/jbc.270.30.18133. [DOI] [PubMed] [Google Scholar]
- 36.Lipfert L, Haimovich B, Schaller M D, Cobb B S, Parsons J T, Brugge J S. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miranti C K, Leng L, Maschberger P, Brugge J S, Shattil S J. Curr Biol. 1998;8:1289–1299. doi: 10.1016/s0960-9822(07)00559-3. [DOI] [PubMed] [Google Scholar]
- 38.Savage B, Shattil S J, Ruggeri Z M. J Biol Chem. 1992;267:11300–11306. [PubMed] [Google Scholar]
- 39.Clements J L, Ross-Barta S E, Tygrett L T, Waldschmidt T J, Koretzky G A. J Immunol. 1998;161:3880–3889. [PubMed] [Google Scholar]
- 40.Hodivala-Dilke K M, McHugh K P, Tsakiris D A, Rayburn H, Crowley D, Ullman-Cullere M, Ross F P, Coller B S, Teitelbaum S, Hynes R O. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Law D A, DeGuzman F R, Heiser P, Ministri-Madrid K, Killeen N, Phillips D R. Nature (London) 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y P, Djaffar I, Pidard D, Steiner B, Cieutat A M, Caen J P, Rosa J P. Proc Natl Acad Sci USA. 1992;89:10169–10173. doi: 10.1073/pnas.89.21.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R, Shattil S J, Ambruso D R, Newman P J. J Clin Invest. 1997;100:2393–2403. doi: 10.1172/JCI119780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy-Toledano S. Haemostasis. 1999;29:4–15. doi: 10.1159/000022456. [DOI] [PubMed] [Google Scholar]
- 45.Bubeck Wardenburg J, Pappu R, Bu J Y, Mayer B, Chernoff J, Straus D, Chan A C. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 46.Krause M, Sechi A S, Konradt M, Monner D, Gertler F B, Wehland J. J Cell Biol. 2000;149:181–194. doi: 10.1083/jcb.149.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cichowski K, Brugge J S, Brass L F. J Biol Chem. 1996;271:7544–7550. doi: 10.1074/jbc.271.13.7544. [DOI] [PubMed] [Google Scholar]