Abstract
OBJECTIVE—To estimate muscle metabolism and oxygen delivery to skeletal muscle in patients with chronic heart failure. METHODS—13 patients with chronic heart failure and 15 controls performed calf plantar flexion for six minutes at a constant workload of 50% of one repetition maximum. During recovery from exercise, skeletal muscle content of oxygenated haemoglobin (oxy-Hb) and the level of phosphocreatine (PCr) were measured by near-infrared spectroscopy and 31P-magnetic resonance spectroscopy, respectively. RESULTS—The mean (SD) time constants of PCr and oxy-Hb during recovery from exercise were significantly greater in patients with chronic heart failure than in normal subjects (τ PCr: 76.3 (30.2) s v 36.5 (5.8) s; τ oxy-Hb: 48.3 (7.3) s v 30.1 (7.7) s; p < 0.01). Both time constants were similar in normal subjects, while the τ PCr was significantly greater than the τ oxy-Hb in patients with chronic heart failure. CONCLUSIONS—The slower recovery of PCr compared with oxy-Hb in patients with chronic heart failure indicates that haemoglobin resaturation is not a major rate limiting factor of PCr resynthesis. It is suggested that muscle metabolic recovery may depend more on oxygen utilisation than on haemoglobin resaturation or oxygen delivery in patients with chronic heart failure. Keywords: near-infrared spectroscopy; 31P-magnetic resonance spectroscopy; chronic heart failure; exercise tolerance
Full Text
The Full Text of this article is available as a PDF (145.2 KB).
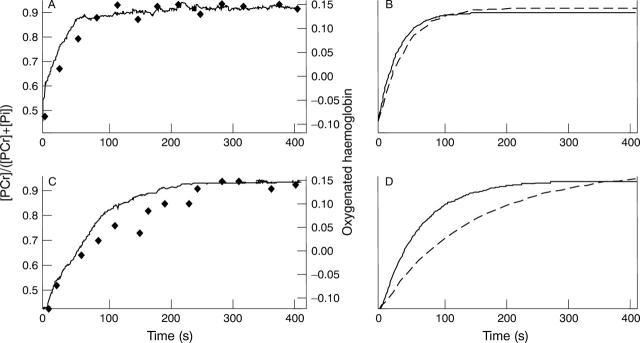
Figure 1 .
Representative spectra showing recovery of phosphocreatine (PCr, filled symbols) and oxygenated haemoglobin (oxy-Hb, solid line) in normal subjects (A) and patients with chronic heart failure (C). Each dataset is fitted with a single exponential curve (B and D). A dotted line indicates the fitting curve of PCr and a solid line shows that of oxy-Hb. The time constants are as follows: B (normal subject): τ oxy-Hb = 28 s; τ PCr = 33 s. D (patient with chronic heart failure): τ oxy-Hb = 53 s; τ PCr = 110 s.τ oxy-Hb, time constant for oxy-Hb resaturation; τ PCr, time constant for PCr resynthesis.
Figure 2 .
Relation between the time constants and the anaerobic threshold in patients with chronic heart failure (filled circles) and normal subjects (empty circles). Left panel shows correlation between anaerobic threshold and τ PCr and right panel shows anaerobic threshold and τ oxy-Hb.
Figure 3 .
The relation between τ PCr and τ oxy-Hb presented by two dimensional plotting. Empty circles indicate normal subjects and filled circles indicate patients with chronic heart failure.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnolda L., Conway M., Dolecki M., Sharif H., Rajagopalan B., Ledingham J. G., Sleight P., Radda G. K. Skeletal muscle metabolism in heart failure: a 31P nuclear magnetic resonance spectroscopy study of leg muscle. Clin Sci (Lond) 1990 Dec;79(6):583–589. doi: 10.1042/cs0790583. [DOI] [PubMed] [Google Scholar]
- Beaver W. L., Wasserman K., Whipp B. J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986 Jun;60(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Belardinelli R., Barstow T. J., Nguyen P., Wasserman K. Skeletal muscle oxygenation and oxygen uptake kinetics following constant work rate exercise in chronic congestive heart failure. Am J Cardiol. 1997 Nov 15;80(10):1319–1324. doi: 10.1016/s0002-9149(97)00672-3. [DOI] [PubMed] [Google Scholar]
- Chance B., Dait M. T., Zhang C., Hamaoka T., Hagerman F. Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am J Physiol. 1992 Mar;262(3 Pt 1):C766–C775. doi: 10.1152/ajpcell.1992.262.3.C766. [DOI] [PubMed] [Google Scholar]
- Di Prampero P. E., Margaria R. Mechanical efficiency of phosphagen (ATP+CP) splitting and its speed of resynthesis. Pflugers Arch. 1969;308(3):197–202. doi: 10.1007/BF00586553. [DOI] [PubMed] [Google Scholar]
- Mancini D. M., Coyle E., Coggan A., Beltz J., Ferraro N., Montain S., Wilson J. R. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989 Nov;80(5):1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- Mancini D. M., Ferraro N., Tuchler M., Chance B., Wilson J. R. Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus-31 nuclear magnetic resonance. Am J Cardiol. 1988 Dec 1;62(17):1234–1240. doi: 10.1016/0002-9149(88)90266-4. [DOI] [PubMed] [Google Scholar]
- Mancini D. M., Walter G., Reichek N., Lenkinski R., McCully K. K., Mullen J. L., Wilson J. R. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992 Apr;85(4):1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- Massie B. M., Conway M., Rajagopalan B., Yonge R., Frostick S., Ledingham J., Sleight P., Radda G. Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure. Evidence for abnormalities unrelated to blood flow. Circulation. 1988 Aug;78(2):320–326. doi: 10.1161/01.cir.78.2.320. [DOI] [PubMed] [Google Scholar]
- Massie B., Conway M., Yonge R., Frostick S., Ledingham J., Sleight P., Radda G., Rajagopalan B. Skeletal muscle metabolism in patients with congestive heart failure: relation to clinical severity and blood flow. Circulation. 1987 Nov;76(5):1009–1019. doi: 10.1161/01.cir.76.5.1009. [DOI] [PubMed] [Google Scholar]
- Matsui S., Tamura N., Hirakawa T., Kobayashi S., Takekoshi N., Murakami E. Assessment of working skeletal muscle oxygenation in patients with chronic heart failure. Am Heart J. 1995 Apr;129(4):690–695. doi: 10.1016/0002-8703(95)90317-8. [DOI] [PubMed] [Google Scholar]
- McCully K. K., Halber C., Posner J. D. Exercise-induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. J Gerontol. 1994 May;49(3):B128–B134. doi: 10.1093/geronj/49.3.b128. [DOI] [PubMed] [Google Scholar]
- McCully K. K., Iotti S., Kendrick K., Wang Z., Posner J. D., Leigh J., Jr, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol (1985) 1994 Jul;77(1):5–10. doi: 10.1152/jappl.1994.77.1.5. [DOI] [PubMed] [Google Scholar]
- Meyer R. A. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988 Apr;254(4 Pt 1):C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Nishijima H., Nishida M., Anzai T., Yonezawa K., Fukuda H., Sato I., Yasuda H. A simple ergometer for 31P NMR spectroscopy during dynamic forearm exercise in a whole body magnetic resonance imaging system. Jpn Heart J. 1992 Mar;33(2):185–192. doi: 10.1536/ihj.33.185. [DOI] [PubMed] [Google Scholar]
- Okita K., Yonezawa K., Nishijima H., Hanada A., Ohtsubo M., Kohya T., Murakami T., Kitabatake A. Skeletal muscle metabolism limits exercise capacity in patients with chronic heart failure. Circulation. 1998 Nov 3;98(18):1886–1891. doi: 10.1161/01.cir.98.18.1886. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Styles P., Matthews P. M., Arnold D. A., Gadian D. G., Bore P., Radda G. K. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986 Feb;3(1):44–54. doi: 10.1002/mrm.1910030107. [DOI] [PubMed] [Google Scholar]
- Walter G., Vandenborne K., McCully K. K., Leigh J. S. Noninvasive measurement of phosphocreatine recovery kinetics in single human muscles. Am J Physiol. 1997 Feb;272(2 Pt 1):C525–C534. doi: 10.1152/ajpcell.1997.272.2.C525. [DOI] [PubMed] [Google Scholar]
- Wiener D. H., Fink L. I., Maris J., Jones R. A., Chance B., Wilson J. R. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation. 1986 Jun;73(6):1127–1136. doi: 10.1161/01.cir.73.6.1127. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Fink L., Maris J., Ferraro N., Power-Vanwart J., Eleff S., Chance B. Evaluation of energy metabolism in skeletal muscle of patients with heart failure with gated phosphorus-31 nuclear magnetic resonance. Circulation. 1985 Jan;71(1):57–62. doi: 10.1161/01.cir.71.1.57. [DOI] [PubMed] [Google Scholar]