Abstract
OBJECTIVE—To improve the biocompatibility of stents using a phosphorylcholine coated stent as a form of biomimicry. INTERVENTIONS—Implantation of phosphorylcholine coated (n = 20) and non-coated (n = 21) stents was performed in the coronary arteries of 25 pigs. The animals were killed after five days (n = 6), four weeks (n = 7), and 12 weeks (n = 8), and the vessels harvested for histology, scanning electron microscopy, and morphometry. MAIN OUTCOME MEASURES—Stent performance was assessed by studying early endothelialisation, neointima formation, and vessel wall reaction to the synthetic coating. RESULTS—Stent thrombosis did not occur in either group. Morphometry showed no significant differences between the two study groups at any time point. At five days both the coated and non-coated stents were equally well endothelialised (91% v 92%, respectively). At four and 12 weeks there was no difference in intimal thickness between the coated and non-coated stents. Up to 12 weeks postimplant the phosphorylcholine coating was still discernible in the stent strut voids, and did not appear to elicit an adverse inflammatory response. CONCLUSION—In this animal model the phosphorylcholine coating showed excellent blood and tissue compatibility, unlike a number of other polymers tested in a similar setting. Given that the coating was present up to 12 weeks postimplant with no adverse tissue reaction, it may be a potential candidate polymer for local drug delivery. Keywords: phosphorylcholine; stents; coatings; biocompatible materials
Full Text
The Full Text of this article is available as a PDF (218.4 KB).
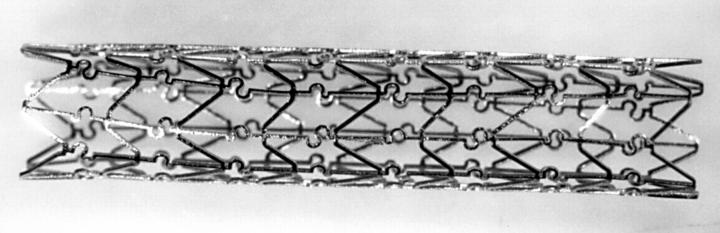
Figure 1 .
A macroscopic view of the divYsio stent.
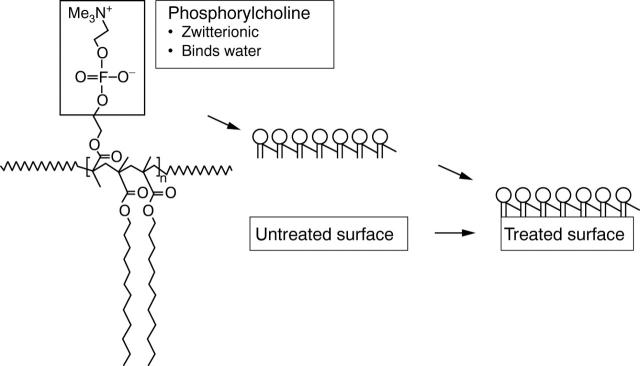
Figure 2 .
Schematic representation of the phosphorylcholine coating applied to the divYsio stent (modified from Campbell and colleagues8).
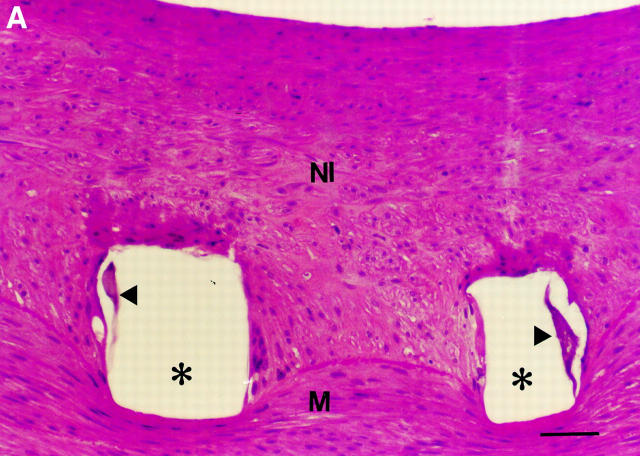
Figure 3 .
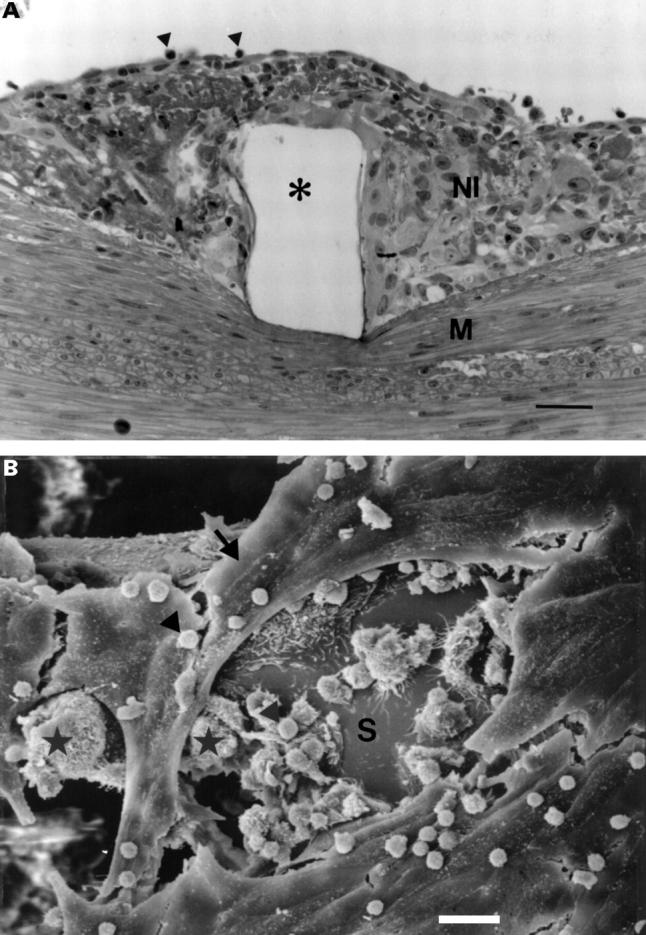
(A) Light microscopy of the intimal thickening at five days postimplant, showing granulation tissue over the stent wire void (*), with occasional leucocytes (arrowheads) attached to the endothelium (bar = 30 µm). NI, neointima; M, media; haematoxylin and eosin. (B) SEM of a stent strut (S) at five days, showing an incomplete endothelial lining (arrow), with leucocytes (arrowheads) and macrophages (*) occupying areas devoid of endothelium (bar = 20 µm).
Figure 4 .
Overview (left) of a stented artery at four weeks showing an asymmetric neointima (bar = 500 µm), with detail (right) showing cells in a haphazard orientation with thrombus remnants (arrowhead) in the intimal/medial border zone (bar = 100 µm). NI, neointima; M, media; A, adventitia; haematoxylin and eosin.
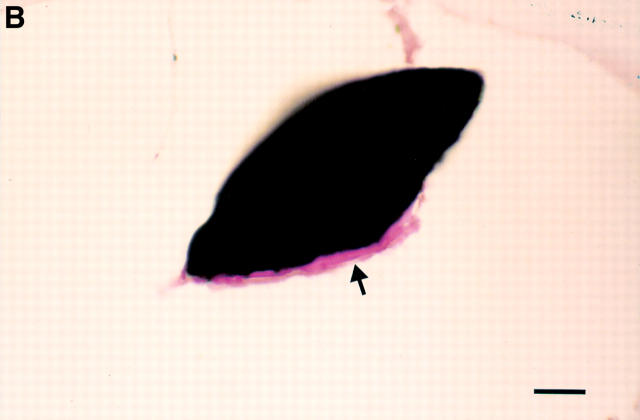
Figure 5 .
(A) Detail of purple stained coating (arrowheads) observed in the stent strut void (*) 12 weeks after implantation of a phosphorylcholine coated stent (bar = 45 µm). NI, neointima; M, media; haematoxylin and eosin. (B) Detail of the cut surface of a stent strut from a non-implanted coated stent (bar = 20 µm). The phosphorylcholine coating (arrow) seen at the strut edge is similar in appearance to that seen in A. Haematoxylin and eosin.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal R. K., Ireland D. C., Azrin M. A., Ezekowitz M. D., de Bono D. P., Gershlick A. H. Antithrombotic potential of polymer-coated stents eluting platelet glycoprotein IIb/IIIa receptor antibody. Circulation. 1996 Dec 15;94(12):3311–3317. doi: 10.1161/01.cir.94.12.3311. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., O'Byrne V., Stratford P. W., Quirk I., Vick T. A., Wiles M. C., Yianni Y. P. Biocompatible surfaces using methacryloylphosphorylcholine laurylmethacrylate copolymer. ASAIO J. 1994 Jul-Sep;40(3):M853–M857. doi: 10.1097/00002480-199407000-00118. [DOI] [PubMed] [Google Scholar]
- Chen C., Lumsden A. B., Ofenloch J. C., Noe B., Campbell E. J., Stratford P. W., Yianni Y. P., Taylor A. S., Hanson S. R. Phosphorylcholine coating of ePTFE grafts reduces neointimal hyperplasia in canine model. Ann Vasc Surg. 1997 Jan;11(1):74–79. doi: 10.1007/s100169900013. [DOI] [PubMed] [Google Scholar]
- De Scheerder I. K., Wilczek K. L., Verbeken E. V., Vandorpe J., Lan P. N., Schacht E., De Geest H., Piessens J. Biocompatibility of polymer-coated oversized metallic stents implanted in normal porcine coronary arteries. Atherosclerosis. 1995 Apr 7;114(1):105–114. doi: 10.1016/0021-9150(94)05472-u. [DOI] [PubMed] [Google Scholar]
- De Scheerder I. K., Wilczek K. L., Verbeken E. V., Vandorpe J., Lan P. N., Schacht E., Piessens J., De Geest H. Biocompatibility of biodegradable and nonbiodegradable polymer-coated stents implanted in porcine peripheral arteries. Cardiovasc Intervent Radiol. 1995 Jul-Aug;18(4):227–232. doi: 10.1007/BF00239417. [DOI] [PubMed] [Google Scholar]
- Dev V., Eigler N., Sheth S., Lambert T., Forrester J., Litvack F. Kinetics of drug delivery to the arterial wall via polyurethane-coated removable nitinol stent: comparative study of two drugs. Cathet Cardiovasc Diagn. 1995 Mar;34(3):272–278. doi: 10.1002/ccd.1810340124. [DOI] [PubMed] [Google Scholar]
- Fontaine A. B., Koelling K., Passos S. D., Cearlock J., Hoffman R., Spigos D. G. Polymeric surface modifications of tantalum stents. J Endovasc Surg. 1996 Aug;3(3):276–283. doi: 10.1177/152660289600300306. [DOI] [PubMed] [Google Scholar]
- Goods C. M., Al-Shaibi K. F., Yadav S. S., Liu M. W., Negus B. H., Iyer S. S., Dean L. S., Jain S. P., Baxley W. A., Parks J. M. Utilization of the coronary balloon-expandable coil stent without anticoagulation or intravascular ultrasound. Circulation. 1996 May 15;93(10):1803–1808. doi: 10.1161/01.cir.93.10.1803. [DOI] [PubMed] [Google Scholar]
- Hunter S., Angelini G. D. Phosphatidylcholine-coated chest tubes improve drainage after open heart operation. Ann Thorac Surg. 1993 Dec;56(6):1339–1342. doi: 10.1016/0003-4975(93)90678-b. [DOI] [PubMed] [Google Scholar]
- Hårdhammar P. A., van Beusekom H. M., Emanuelsson H. U., Hofma S. H., Albertsson P. A., Verdouw P. D., Boersma E., Serruys P. W., van der Giessen W. J. Reduction in thrombotic events with heparin-coated Palmaz-Schatz stents in normal porcine coronary arteries. Circulation. 1996 Feb 1;93(3):423–430. doi: 10.1161/01.cir.93.3.423. [DOI] [PubMed] [Google Scholar]
- Ikada Y. Surface modification of polymers for medical applications. Biomaterials. 1994 Aug;15(10):725–736. doi: 10.1016/0142-9612(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Keane D., Haase J., Slager C. J., Montauban van Swijndregt E., Lehmann K. G., Ozaki Y., di Mario C., Kirkeeide R., Serruys P. W. Comparative validation of quantitative coronary angiography systems. Results and implications from a multicenter study using a standardized approach. Circulation. 1995 Apr 15;91(8):2174–2183. doi: 10.1161/01.cir.91.8.2174. [DOI] [PubMed] [Google Scholar]
- Kuiper K. K., Robinson K. A., Chronos N. A., Cui J., Palmer S. J., Nordrehaug J. E. Phosphorylcholine-coated metallic stents in rabbit iliac and porcine coronary arteries. Scand Cardiovasc J. 1998;32(5):261–268. doi: 10.1080/14017439850139843. [DOI] [PubMed] [Google Scholar]
- Kullo I. J., Mozes G., Schwartz R. S., Gloviczki P., Crotty T. B., Barber D. A., Katusic Z. S., O'Brien T. Adventitial gene transfer of recombinant endothelial nitric oxide synthase to rabbit carotid arteries alters vascular reactivity. Circulation. 1997 Oct 7;96(7):2254–2261. doi: 10.1161/01.cir.96.7.2254. [DOI] [PubMed] [Google Scholar]
- Lambert T. L., Dev V., Rechavia E., Forrester J. S., Litvack F., Eigler N. L. Localized arterial wall drug delivery from a polymer-coated removable metallic stent. Kinetics, distribution, and bioactivity of forskolin. Circulation. 1994 Aug;90(2):1003–1011. doi: 10.1161/01.cir.90.2.1003. [DOI] [PubMed] [Google Scholar]
- Lincoff A. M., Furst J. G., Ellis S. G., Tuch R. J., Topol E. J. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol. 1997 Mar 15;29(4):808–816. doi: 10.1016/s0735-1097(96)00584-0. [DOI] [PubMed] [Google Scholar]
- Moussa I., Di Mario C., Di Francesco L., Reimers B., Blengino S., Colombo A. Subacute stent thrombosis and the anticoagulation controversy: changes in drug therapy, operator technique, and the impact of intravascular ultrasound. Am J Cardiol. 1996 Aug 14;78(3A):13–17. doi: 10.1016/s0002-9149(96)00486-9. [DOI] [PubMed] [Google Scholar]
- Murphy J. G., Schwartz R. S., Edwards W. D., Camrud A. R., Vlietstra R. E., Holmes D. R., Jr Percutaneous polymeric stents in porcine coronary arteries. Initial experience with polyethylene terephthalate stents. Circulation. 1992 Nov;86(5):1596–1604. doi: 10.1161/01.cir.86.5.1596. [DOI] [PubMed] [Google Scholar]
- Reiber J. H., Serruys P. W., Kooijman C. J., Wijns W., Slager C. J., Gerbrands J. J., Schuurbiers J. C., den Boer A., Hugenholtz P. G. Assessment of short-, medium-, and long-term variations in arterial dimensions from computer-assisted quantitation of coronary cineangiograms. Circulation. 1985 Feb;71(2):280–288. doi: 10.1161/01.cir.71.2.280. [DOI] [PubMed] [Google Scholar]
- Rogers C., Edelman E. R. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation. 1995 Jun 15;91(12):2995–3001. doi: 10.1161/01.cir.91.12.2995. [DOI] [PubMed] [Google Scholar]
- Wilczek K., Scheerder I. D., Wang K., Verbeken E., Piessens J. Comparison of self-expanding polyethylene terephthalate and metallic stents implanted in porcine iliac arteries. Cardiovasc Intervent Radiol. 1996 May-Jun;19(3):176–180. doi: 10.1007/BF02577615. [DOI] [PubMed] [Google Scholar]
- de Scheerder I., Wang K., Wilczek K., van Dorpe J., Verbeken E., Desmet W., Schacht E., Piessens J. Local methylprednisolone inhibition of foreign body response to coated intracoronary stents. Coron Artery Dis. 1996 Feb;7(2):161–166. doi: 10.1097/00019501-199602000-00011. [DOI] [PubMed] [Google Scholar]
- van Wachem P. B., Vreriks C. M., Beugeling T., Feijen J., Bantjes A., Detmers J. P., van Aken W. G. The influence of protein adsorption on interactions of cultured human endothelial cells with polymers. J Biomed Mater Res. 1987 Jun;21(6):701–718. doi: 10.1002/jbm.820210603. [DOI] [PubMed] [Google Scholar]
- van der Giessen W. J., Lincoff A. M., Schwartz R. S., van Beusekom H. M., Serruys P. W., Holmes D. R., Jr, Ellis S. G., Topol E. J. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996 Oct 1;94(7):1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- van der Giessen W. J., Serruys P. W., van Beusekom H. M., van Woerkens L. J., van Loon H., Soei L. K., Strauss B. H., Beatt K. J., Verdouw P. D. Coronary stenting with a new, radiopaque, balloon-expandable endoprosthesis in pigs. Circulation. 1991 May;83(5):1788–1798. doi: 10.1161/01.cir.83.5.1788. [DOI] [PubMed] [Google Scholar]
- von Segesser L. K., Olah A., Leskosek B., Turina M. Coagulation patterns in bovine left heart bypass with phospholipid versus heparin surface coating. ASAIO J. 1993 Jan-Mar;39(1):43–46. [PubMed] [Google Scholar]