Abstract
OBJECTIVE—To investigate the clinical significance of circulating hepatocyte growth factor (HGF) and the role of peripheral blood mononuclear cells (monocytes), which are a possible source of HGF, in patients with acute myocardial infarction. DESIGN AND PATIENTS—37 patients with acute myocardial infarction and 13 normal control subjects were recruited. Peripheral venous blood samples were drawn from the infarct patients 1, 7, 14, and 21 days after onset. Monocytes were isolated from peripheral blood at those times. HGF concentrations in serum and in a culture medium of monocytes after incubation for 24 hours (monocyte HGF levels) were measured by enzyme linked immunosorbent assay. RESULTS—Serum HGF and monocyte HGF values within seven days after onset of myocardial infarction were significantly higher than those of control subjects and decreased by day 14. There were significant positive correlations between serum HGF and monocyte HGF levels on day 7; between maximum plasma creatine phosphokinase levels and serum HGF levels on day 1; between maximum plasma C reactive protein and serum HGF levels; and between maximum C reactive protein and monocyte HGF levels. Monocyte HGF levels were raised in the patients with progression of ventricular enlargement in the course of acute myocardial infarction. CONCLUSIONS—Early serum HGF concentrations reflect the extent of myocardial damage in acute myocardial infarction patients. Inflammation after acute myocardial infarction is supposed to be involved in enhanced HGF production. Monocytes may play an important role in ventricular remodelling after acute myocardial infarction by releasing the cardiovascular protective mitogen HGF. Keywords: C reactive protein; cytokines; ischaemic heart disease; remodelling; hepatocyte growth factor
Full Text
The Full Text of this article is available as a PDF (143.7 KB).
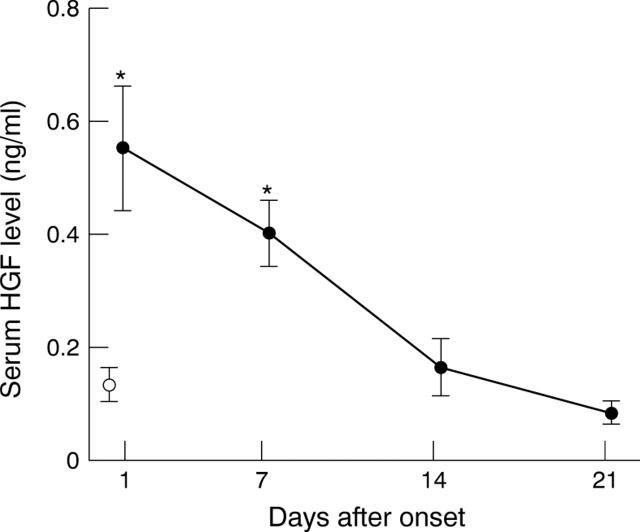
Figure 1 .
Time course of serum hepatocyte growth factor (HGF) concentrations in patients with acute myocardial infarction. HGF was determined by enzyme linked immunosorbent assay as described in Methods. Serum HGF reached a peak on day 1 and decreased by day 21. Serum HGF in the patients was significantly higher than in the normal controls on day 1 (0.54 (0.11) v 0.12 (0.03) ng/ml, p < 0.05), and remained significantly higher on day 7. Filled circles, patients with acute myocardial infarction; empty circles, control subjects. Error bars = SEM. *p < 0.05 v control subjects.
Figure 2 .

(A) Hepatocyte growth factor (HGF) concentrations in the supernatant of cultured peripheral blood mononuclear cells (monocytes) in patients with acute myocardial infarction and in control subjects. Monocytes were isolated and cultured as described in Methods. HGF levels in the monocyte culture medium increased time dependently until 24 hours after incubation. (B) Changes in HGF production by monocytes in the course of acute myocardial infarction. HGF levels in the monocyte culture medium after incubation for 24 hours are called "monocyte HGF levels" and used as a marker of the HGF producing ability of monocytes. Monocyte HGF levels were significantly higher than those of control subjects on day 1 but decreased by day 14. Filled circles, patients with acute myocardial infarction; empty circles, control subjects. Error bars = SEM. *p < 0.05; **p < 0.01 v control subjects.
Figure 3 .

Correlations between serum hepatocyte growth factor (HGF) levels and monocyte HGF levels in patients with acute myocardial infarction: a weak correlation was found between serum HGF and monocyte HGF on day 1 after onset of (panel A, r = +0.47, p < 0.05), and a positive correlation was found between these two variables on day 7 after onset (panel B, r = +0.54, p < 0.01).
Figure 4 .

Correlations between maximum creatine phosphokinase (CPK) and hepatocyte growth factor (HGF): there was a significant positive correlation between maximum plasma CPK and both maximum serum HGF (panel A, r = +0.54, p < 0.01) and serum HGF on day 1 (panel B, r = +0.61, p < 0.001).
Figure 5 .

Correlations between maximum C reactive protein levels and hepatocyte growth factor (HGF) levels: there was a significant positive correlation between maximum plasma C reactive protein and maximum serum HGF (panel A, r = +0.55, p < 0.01), and between maximum C reactive protein and maximum monocyte HGF (panel B, r = +0.65, p < 0.001).
Figure 6 .
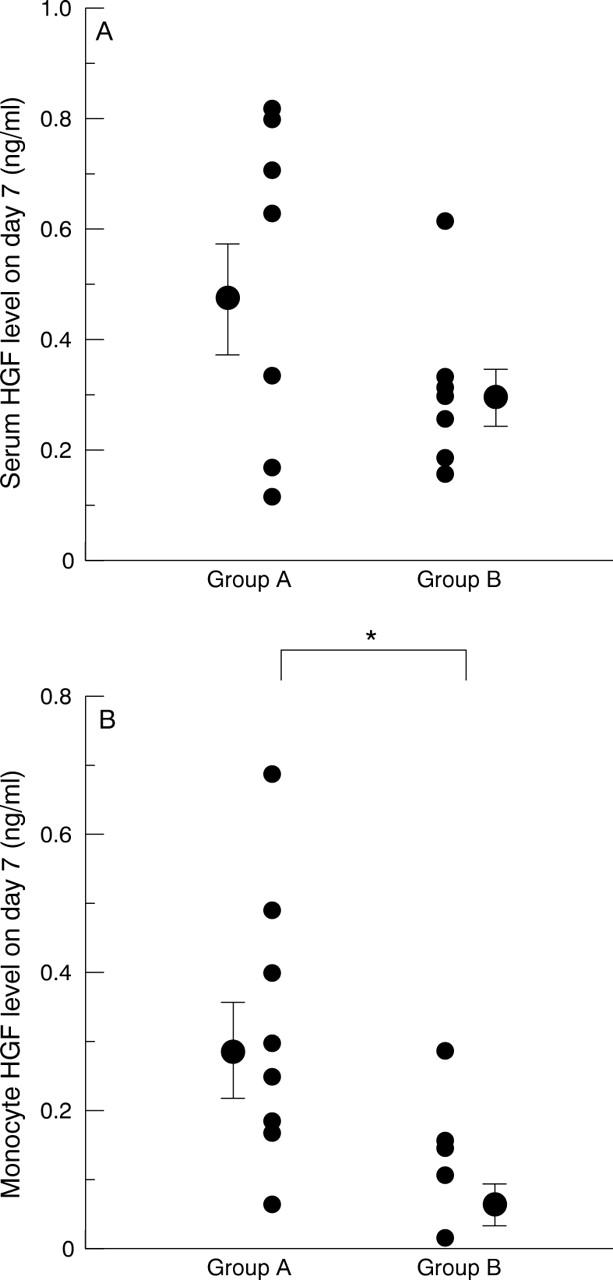
Hepatocyte growth factor (HGF) levels in the patients with or without left ventricular dilatation in the course of acute myocardial infarction. We divided the patients into two groups according to the changes in left ventricular end diastolic volume index (LVEDVI) (group A: eight patients with increases in LVEDVI during the course of acute myocardial infarction; group B: eight patients without increases in LVEDVI). (A) Mean serum HGF on day 7 in group A was higher than in group B, but the difference was not significant (0.47 (0.10) v 0.29 (0.05) ng/ml, p = 0.13). (B) Monocyte HGF levels on day 7 in group A were significantly higher than in group B (0.30 (0.07) v 0.08 (0.03) ng/ml, p < 0.05). Error bars = SEM. *p < 0.05.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boros P., Miller C. M. Hepatocyte growth factor: a multifunctional cytokine. Lancet. 1995 Feb 4;345(8945):293–295. doi: 10.1016/s0140-6736(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Di Renzo M. F., Ziche M., Bocchietto E., Olivero M., Naldini L., Gaudino G., Tamagnone L., Coffer A., Comoglio P. M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992 Nov;119(3):629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., White F. C., Roth D. M., Bloor C. M. Heparin accelerates coronary collateral development in a porcine model of coronary artery occlusion. Circulation. 1993 Jul;88(1):198–207. doi: 10.1161/01.cir.88.1.198. [DOI] [PubMed] [Google Scholar]
- Gillum R. F., Fortmann S. P., Prineas R. J., Kottke T. E. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984 Jul;108(1):150–158. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Kleinman H. K., Goldberg I. D., Bhargava M. M., Nickoloff B. J., Kinsella J. L., Polverini P., Rosen E. M. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanoue M., Kawaida K., Takao S., Shimazu H., Noji S., Matsumoto K., Nakamura T. Rapid and marked induction of hepatocyte growth factor during liver regeneration after ischemic or crush injury. Hepatology. 1992 Dec;16(6):1485–1492. doi: 10.1002/hep.1840160626. [DOI] [PubMed] [Google Scholar]
- Haverkate F., Thompson S. G., Pyke S. D., Gallimore J. R., Pepys M. B. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997 Feb 15;349(9050):462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- Igawa T., Matsumoto K., Kanda S., Saito Y., Nakamura T. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am J Physiol. 1993 Jul;265(1 Pt 2):F61–F69. doi: 10.1152/ajprenal.1993.265.1.F61. [DOI] [PubMed] [Google Scholar]
- Lagrand W. K., Niessen H. W., Wolbink G. J., Jaspars L. H., Visser C. A., Verheugt F. W., Meijer C. J., Hack C. E. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997 Jan 7;95(1):97–103. doi: 10.1161/01.cir.95.1.97. [DOI] [PubMed] [Google Scholar]
- Liuzzo G., Biasucci L. M., Gallimore J. R., Grillo R. L., Rebuzzi A. G., Pepys M. B., Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994 Aug 18;331(7):417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- Matsumori A., Furukawa Y., Hashimoto T., Ono K., Shioi T., Okada M., Iwasaki A., Nishio R., Sasayama S. Increased circulating hepatocyte growth factor in the early stage of acute myocardial infarction. Biochem Biophys Res Commun. 1996 Apr 16;221(2):391–395. doi: 10.1006/bbrc.1996.0606. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Shimomura T., Naka D., Kitamura N. Proteolytic activation of hepatocyte growth factor in response to tissue injury. J Biol Chem. 1994 Mar 25;269(12):8966–8970. [PubMed] [Google Scholar]
- Morishita R., Nakamura S., Nakamura Y., Aoki M., Moriguchi A., Kida I., Yo Y., Matsumoto K., Nakamura T., Higaki J. Potential role of an endothelium-specific growth factor, hepatocyte growth factor, on endothelial damage in diabetes. Diabetes. 1997 Jan;46(1):138–142. doi: 10.2337/diab.46.1.138. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Moriguchi A., Morishita R., Aoki M., Yo Y., Hayashi S., Nakano N., Katsuya T., Nakata S., Takami S. A novel vascular modulator, hepatocyte growth factor (HGF), as a potential index of the severity of hypertension. Biochem Biophys Res Commun. 1998 Jan 6;242(1):238–243. doi: 10.1006/bbrc.1997.7800. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nawa K., Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Teramoto H., Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Teramoto H., Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Morishita R., Higaki J., Kida I., Aoki M., Moriguchi A., Yamada K., Hayashi S., Yo Y., Matsumoto K. Expression of local hepatocyte growth factor system in vascular tissues. Biochem Biophys Res Commun. 1995 Oct 13;215(2):483–488. doi: 10.1006/bbrc.1995.2490. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Morishita R., Higaki J., Kida I., Aoki M., Moriguchi A., Yamada K., Hayashi S., Yo Y., Nakano H. Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. J Hypertens. 1996 Sep;14(9):1067–1072. doi: 10.1097/00004872-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Morishita R., Nakamura S., Aoki M., Moriguchi A., Matsumoto K., Nakamura T., Higaki J., Ogihara T. A vascular modulator, hepatocyte growth factor, is associated with systolic pressure. Hypertension. 1996 Sep;28(3):409–413. doi: 10.1161/01.hyp.28.3.409. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Ushiyama M., Nanbu A., Ohtsuka K., Takahashi H., Yoshimura M. Serum hepatocyte growth factor as a possible indicator of arteriosclerosis. J Hypertens. 1997 Oct;15(10):1137–1142. doi: 10.1097/00004872-199715100-00011. [DOI] [PubMed] [Google Scholar]
- Ohira H., Miyata M., Kuroda M., Takagi T., Tojo J., Ochiai H., Kokubun M., Nishimaki T., Kasukawa R., Obara K. Interleukin-6 induces proliferation of rat hepatocytes in vivo. J Hepatol. 1996 Dec;25(6):941–947. doi: 10.1016/s0168-8278(96)80300-x. [DOI] [PubMed] [Google Scholar]
- Ono K., Matsumori A., Shioi T., Furukawa Y., Sasayama S. Enhanced expression of hepatocyte growth factor/c-Met by myocardial ischemia and reperfusion in a rat model. Circulation. 1997 Jun 3;95(11):2552–2558. doi: 10.1161/01.cir.95.11.2552. [DOI] [PubMed] [Google Scholar]
- Pietilä K. O., Harmoinen A. P., Jokiniitty J., Pasternack A. I. Serum C-reactive protein concentration in acute myocardial infarction and its relationship to mortality during 24 months of follow-up in patients under thrombolytic treatment. Eur Heart J. 1996 Sep;17(9):1345–1349. doi: 10.1093/oxfordjournals.eurheartj.a015068. [DOI] [PubMed] [Google Scholar]
- Ridker P. M., Cushman M., Stampfer M. J., Tracy R. P., Hennekens C. H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997 Apr 3;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Nigam S. K., Goldberg I. D. Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol. 1994 Dec;127(6 Pt 2):1783–1787. doi: 10.1083/jcb.127.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Bottaro D. P., Aaronson S. A. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta. 1993 Dec 23;1155(3):357–371. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- Sasayama S., Fujita M. Recent insights into coronary collateral circulation. Circulation. 1992 Mar;85(3):1197–1204. doi: 10.1161/01.cir.85.3.1197. [DOI] [PubMed] [Google Scholar]
- Tamura M., Arakaki N., Tsubouchi H., Takada H., Daikuhara Y. Enhancement of human hepatocyte growth factor production by interleukin-1 alpha and -1 beta and tumor necrosis factor-alpha by fibroblasts in culture. J Biol Chem. 1993 Apr 15;268(11):8140–8145. [PubMed] [Google Scholar]
- Taniguchi T., Toi M., Tominaga T. Rapid induction of hepatocyte growth factor by heparin. Lancet. 1994 Aug 13;344(8920):470–470. doi: 10.1016/s0140-6736(94)91797-3. [DOI] [PubMed] [Google Scholar]
- Toss H., Lindahl B., Siegbahn A., Wallentin L. Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. Circulation. 1997 Dec 16;96(12):4204–4210. doi: 10.1161/01.cir.96.12.4204. [DOI] [PubMed] [Google Scholar]
- Zarnegar R., Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989 Jun 15;49(12):3314–3320. [PubMed] [Google Scholar]