Full Text
The Full Text of this article is available as a PDF (172.6 KB).
Figure 1 .
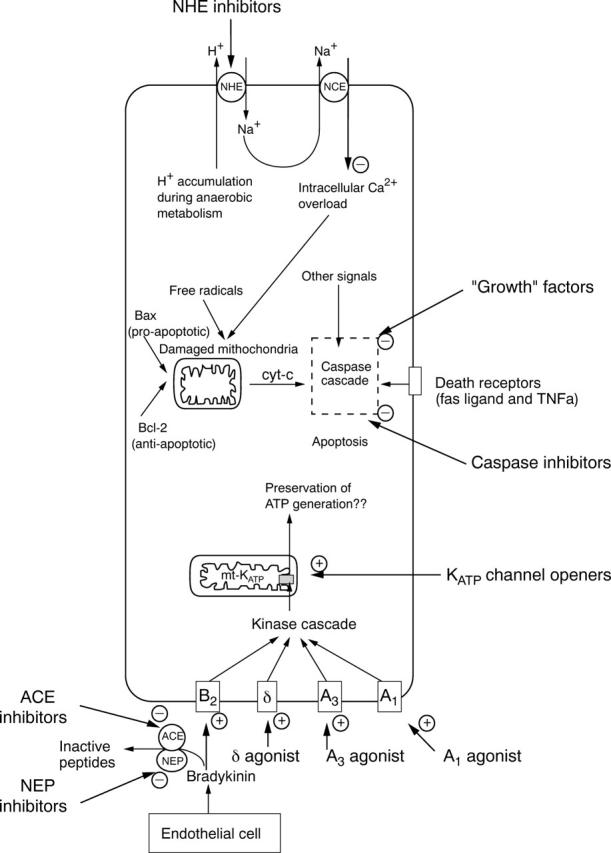
Modification of several molecular targets presents new therapeutic possibilities for limitation or delay of myocyte death during ischaemia and/or reperfusion. The figure summarises three promising approaches to infarct limitation during ischaemia and/or reperfusion. Top Intracellular accumulation of protons during ischaemia leads to the activation of the sarcolemmal sodium-hydrogen exchanger (NHE). Consequent intracellular sodium overload causes the activation of the sarcolemmal sodium-calcium exchanger (NCE) and predisposes to intracellular calcium overload. Inhibitors of NHE attenuate this mechanism of injury and can limit infarct size in experimental models. Bottom Preconditioning is an endogenous adaptive response in myocytes. Mediators released in ischaemic tissue include adenosine, noradrenaline, bradykinin, and opioid peptides. These mediators act via seven transmembrane G-protein coupled receptors on myocytes, (adenosine A1 and A3 receptors, δ-opioid receptors, and bradykinin B2 receptors) and initiate a complex kinase signalling cascade, which opens the KATP channel in the mitochondrial inner membrane (mt-KATP). These channels are the likely end effector of preconditioning. Opening of the channel enhances myocyte viability during ischaemia through a mechanism that is likely to involve preservation of mitochondrial integrity and ATP generating capacity. There are several possibilities for exploitation of pharmacological preconditioning ("preconditioning mimetics"). Adenosine A1 and A3 receptor agonists and δ-opioid receptor agonists activate the preconditioning signal cascade and limit infarct size in experimental models. Potentiation of endogenously generated bradykinin during ischaemia is possible with angiotensin converting enzyme (ACE) inhibitors and neutral endopeptidase (NEP) inhibitors. These agents prevent the breakdown of bradykinin and augment its protective effects on myocytes. Centre The predominant pathological feature of infarction is myocyte death by necrosis. However, apoptosis is now recognised as an additional mechanism of cell death which may be activated, particularly during reperfusion. Regulation of apoptotic pathways is extremely complex and incompletely understood, but inhibition of apoptosis may be a therapeutic route to salvage myocytes during ischaemia and/or reperfusion. Caspase inhibitors and counter regulatory growth factors limit infarct size when administered during the early reperfusion period.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. V., Willerson J. T. Thrombolysis in acute myocardial infarction. N Engl J Med. 1993 Sep 2;329(10):703–709. doi: 10.1056/NEJM199309023291006. [DOI] [PubMed] [Google Scholar]
- Bartling B., Holtz J., Darmer D. Contribution of myocyte apoptosis to myocardial infarction? Basic Res Cardiol. 1998 Apr;93(2):71–84. doi: 10.1007/s003950050065. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985 Nov;76(5):1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge E., Munch-Ellingsen J., Ytrehus K. Reduced infarct size in the rabbit heart in vivo by ethylisopropyl-amiloride. A role for Na+/H+ exchange. Basic Res Cardiol. 1996 May-Jun;91(3):203–209. doi: 10.1007/BF00788906. [DOI] [PubMed] [Google Scholar]
- Carr C. S., Hill R. J., Masamune H., Kennedy S. P., Knight D. R., Tracey W. R., Yellon D. M. Evidence for a role for both the adenosine A1 and A3 receptors in protection of isolated human atrial muscle against simulated ischaemia. Cardiovasc Res. 1997 Oct;36(1):52–59. doi: 10.1016/s0008-6363(97)00160-0. [DOI] [PubMed] [Google Scholar]
- Dana A., Baxter G. F., Walker J. M., Yellon D. M. Prolonging the delayed phase of myocardial protection: repetitive adenosine A1 receptor activation maintains rabbit myocardium in a preconditioned state. J Am Coll Cardiol. 1998 Apr;31(5):1142–1149. doi: 10.1016/s0735-1097(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Endres M., Namura S., Shimizu-Sasamata M., Waeber C., Zhang L., Gómez-Isla T., Hyman B. T., Moskowitz M. A. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab. 1998 Mar;18(3):238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- Fath-Ordoubadi F., Beatt K. J. Glucose-insulin-potassium therapy for treatment of acute myocardial infarction: an overview of randomized placebo-controlled trials. Circulation. 1997 Aug 19;96(4):1152–1156. doi: 10.1161/01.cir.96.4.1152. [DOI] [PubMed] [Google Scholar]
- Fliss H. Accelerated apoptosis in reperfused myocardium: friend of foe? Basic Res Cardiol. 1998 Apr;93(2):90–93. doi: 10.1007/s003950050067. [DOI] [PubMed] [Google Scholar]
- Frölich O., Karmazyn M. The Na-H exchanger revisited: an update on Na-H exchange regulation and the role of the exchanger in hypertension and cardiac function in health and disease. Cardiovasc Res. 1997 Nov;36(2):138–148. doi: 10.1016/s0008-6363(97)00200-9. [DOI] [PubMed] [Google Scholar]
- Garlid K. D., Paucek P., Yarov-Yarovoy V., Murray H. N., Darbenzio R. B., D'Alonzo A. J., Lodge N. J., Smith M. A., Grover G. J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997 Dec;81(6):1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- Goto M., Liu Y., Yang X. M., Ardell J. L., Cohen M. V., Downey J. M. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995 Sep;77(3):611–621. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- Gumina R. J., Mizumura T., Beier N., Schelling P., Schultz J. J., Gross G. J. A new sodium/hydrogen exchange inhibitor, EMD 85131, limits infarct size in dogs when administered before or after coronary artery occlusion. J Pharmacol Exp Ther. 1998 Jul;286(1):175–183. [PubMed] [Google Scholar]
- Heusch G., Rose J., Ehring T. Cardioprotection by ACE inhibitors in myocardial ischaemia/reperfusion. The importance of bradykinin. Drugs. 1997;54 (Suppl 5):31–41. doi: 10.2165/00003495-199700545-00006. [DOI] [PubMed] [Google Scholar]
- Imagawa J., Baxter G. F., Yellon D. M. Myocardial protection afforded by nicorandil and ischaemic preconditioning in a rabbit infarct model in vivo. J Cardiovasc Pharmacol. 1998 Jan;31(1):74–79. doi: 10.1097/00005344-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Karmazyn M. The sodium-hydrogen exchange system in the heart: its role in ischemic and reperfusion injury and therapeutic implications. Can J Cardiol. 1996 Oct;12(10):1074–1082. [PubMed] [Google Scholar]
- Linz W., Albus U., Crause P., Jung W., Weichert A., Schölkens B. A., Scholz W. Dose-dependent reduction of myocardial infarct mass in rabbits by the NHE-1 inhibitor cariporide (HOE 642). Clin Exp Hypertens. 1998 Oct;20(7):733–749. doi: 10.3109/10641969809052116. [DOI] [PubMed] [Google Scholar]
- Liu G. S., Thornton J., Van Winkle D. M., Stanley A. W., Olsson R. A., Downey J. M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991 Jul;84(1):350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- Liu Y., Sato T., O'Rourke B., Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998 Jun 23;97(24):2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Lopez A. D., Murray C. C. The global burden of disease, 1990-2020. Nat Med. 1998 Nov;4(11):1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- Mahaffey K. W., Puma J. A., Barbagelata N. A., DiCarli M. F., Leesar M. A., Browne K. F., Eisenberg P. R., Bolli R., Casas A. C., Molina-Viamonte V. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999 Nov 15;34(6):1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- Mizumura T., Nithipatikom K., Gross G. J. Infarct size-reducing effect of nicorandil is mediated by the KATP channel but not by its nitrate-like properties in dogs. Cardiovasc Res. 1996 Aug;32(2):274–285. doi: 10.1016/0008-6363(96)00061-2. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Reperfusion injury and its pharmacologic modification. Circulation. 1989 Oct;80(4):1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- Parratt J. R. Protection of the heart by ischaemic preconditioning: mechanisms and possibilities for pharmacological exploitation. Trends Pharmacol Sci. 1994 Jan;15(1):19–25. doi: 10.1016/0165-6147(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Parratt J. R., Vegh A., Papp J. G. Bradykinin as an endogenous myocardial protective substance with particular reference to ischemic preconditioning--a brief review of the evidence. Can J Physiol Pharmacol. 1995 Jul;73(7):837–842. doi: 10.1139/y95-114. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Purcell H. J., Fox K. M. Cardioprotection by opening of the K(ATP) channel in unstable angina. Is this a clinical manifestation of myocardial preconditioning? Results of a randomized study with nicorandil. CESAR 2 investigation. Clinical European studies in angina and revascularization. Eur Heart J. 1999 Jan;20(1):51–57. doi: 10.1053/euhj.1998.1354. [DOI] [PubMed] [Google Scholar]
- Przyklenk K., Kloner R. A. Ischemic preconditioning: exploring the paradox. Prog Cardiovasc Dis. 1998 May-Jun;40(6):517–547. doi: 10.1016/s0033-0620(98)80002-9. [DOI] [PubMed] [Google Scholar]
- Schultz J el-J, Hsu A. K., Nagase H., Gross G. J. TAN-67, a delta 1-opioid receptor agonist, reduces infarct size via activation of Gi/o proteins and KATP channels. Am J Physiol. 1998 Mar;274(3 Pt 2):H909–H914. doi: 10.1152/ajpheart.1998.274.3.H909. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Hsu A. K., Gross G. J. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ Res. 1996 Jun;78(6):1100–1104. doi: 10.1161/01.res.78.6.1100. [DOI] [PubMed] [Google Scholar]
- Schultz J. J., Hsu A. K., Gross G. J. Ischemic preconditioning and morphine-induced cardioprotection involve the delta (delta)-opioid receptor in the intact rat heart. J Mol Cell Cardiol. 1997 Aug;29(8):2187–2195. doi: 10.1006/jmcc.1997.0454. [DOI] [PubMed] [Google Scholar]
- Tracey W. R., Magee W., Masamune H., Kennedy S. P., Knight D. R., Buchholz R. A., Hill R. J. Selective adenosine A3 receptor stimulation reduces ischemic myocardial injury in the rabbit heart. Cardiovasc Res. 1997 Feb;33(2):410–415. doi: 10.1016/s0008-6363(96)00240-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida A., Miura T., Tanno M., Nozawa Y., Kita H., Shimamoto K. Time window for the contribution of the delta-opioid receptor to cardioprotection by ischemic preconditioning in the rat heart. Cardiovasc Drugs Ther. 1998 Sep;12(4):365–373. doi: 10.1023/a:1007720801004. [DOI] [PubMed] [Google Scholar]
- Villanueva F. S., Jankowski R. J., Klibanov S., Pina M. L., Alber S. M., Watkins S. C., Brandenburger G. H., Wagner W. R. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998 Jul 7;98(1):1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- Walker D. M., Walker J. M., Pugsley W. B., Pattison C. W., Yellon D. M. Preconditioning in isolated superfused human muscle. J Mol Cell Cardiol. 1995 Jun;27(6):1349–1357. doi: 10.1016/s0022-2828(05)82397-1. [DOI] [PubMed] [Google Scholar]
- Wall T. M., Sheehy R., Hartman J. C. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994 Aug;270(2):681–689. [PubMed] [Google Scholar]
- Wang J., Drake L., Sajjadi F., Firestein G. S., Mullane K. M., Bullough D. A. Dual activation of adenosine A1 and A3 receptors mediates preconditioning of isolated cardiac myocytes. Eur J Pharmacol. 1997 Feb 12;320(2-3):241–248. doi: 10.1016/s0014-2999(96)00901-6. [DOI] [PubMed] [Google Scholar]
- Woods K. L., Fletcher S., Roffe C., Haider Y. Intravenous magnesium sulphate in suspected acute myocardial infarction: results of the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2) Lancet. 1992 Jun 27;339(8809):1553–1558. doi: 10.1016/0140-6736(92)91828-v. [DOI] [PubMed] [Google Scholar]
- Yaoita H., Ogawa K., Maehara K., Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998 Jan 27;97(3):276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- Yellon D. M., Baxter G. F., Garcia-Dorado D., Heusch G., Sumeray M. S. Ischaemic preconditioning: present position and future directions. Cardiovasc Res. 1998 Jan;37(1):21–33. doi: 10.1016/s0008-6363(97)00214-9. [DOI] [PubMed] [Google Scholar]
- Yellon D. M., Downey J. M. Current research views on myocardial reperfusion and reperfusion injury. Cardioscience. 1990 Jun;1(2):89–98. [PubMed] [Google Scholar]


