Abstract
OBJECTIVE—To investigate the contribution of apoptosis in the development of the skeletal myopathy in chronic heart failure. DESIGN—The electrophoretic pattern of myosin heavy chains (MHC), fibre cross sectional area, number of in situ nick end labelling (TUNEL) positive apoptotic myocyte nuclei, and the tissue levels of caspase-3, Bcl-2, and ubiquitin were determined in biopsies taken from the vastus lateralis muscle. The study involved nine patients with severe chronic heart failure caused by ischaemic heart disease and hibernating myocardium and five controls. RESULTS—In chronic heart failure patients the vastus lateralis showed a significant increase of MHC2a and MHC2b and a greater degree of fibre atrophy, as demonstrated by the decreased cross sectional area. There was also an increased number of TUNEL positive apoptotic myocyte nuclei. Tissue concentrations of Bcl-2 were decreased, while those of caspase-3 and ubiquitin were increased. Peak oxygen consumption (VO2) was negatively correlated with the number of TUNEL positive nuclei and the fibre cross sectional area. There was a correlation between the number of apoptotic nuclei and the fibre cross sectional area, but no correlation between myosin heavy chains and number of apoptotic nuclei. CONCLUSIONS—Myocyte apoptosis occurs in the skeletal muscle of patients with chronic heart failure, and its magnitude is associated with the severity of exercise capacity limitation and the degree of muscle atrophy. Muscle atrophy contributes to the limitation of exercise capacity, together with the increased synthesis of fast, more fatiguable myosin heavy chains. Keywords: apoptosis; chronic heart failure; exercise capacity; myosin heavy chains
Full Text
The Full Text of this article is available as a PDF (163.5 KB).
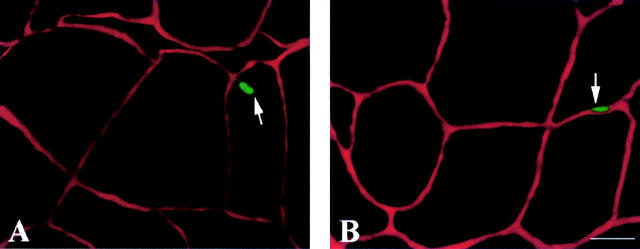
Figure 1 .
(A) TUNEL positive myocyte nucleus. (B) TUNEL positive interstitial nucleus in the vastus lateralis of a patient with chronic heart failure; the interstitial space is stained with laminin. TUNEL, in situ nick end labelling. Bar = 50 µm.
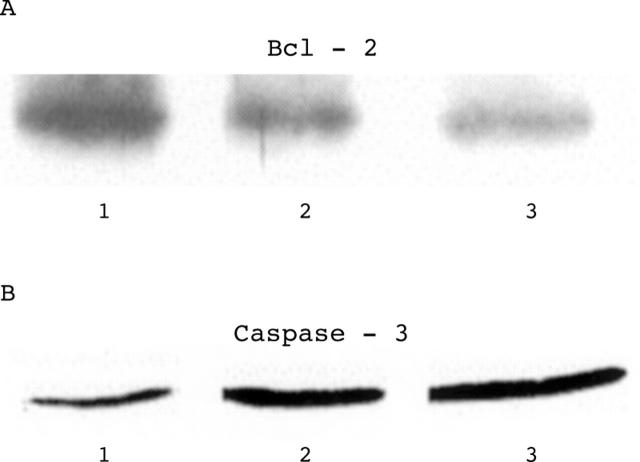
Figure 2 .
Representative western blot of Bcl-2 (panel A) and caspase-3 (panel B). Lane 1, control; lanes 2 and 3, chronic heart failure patients.
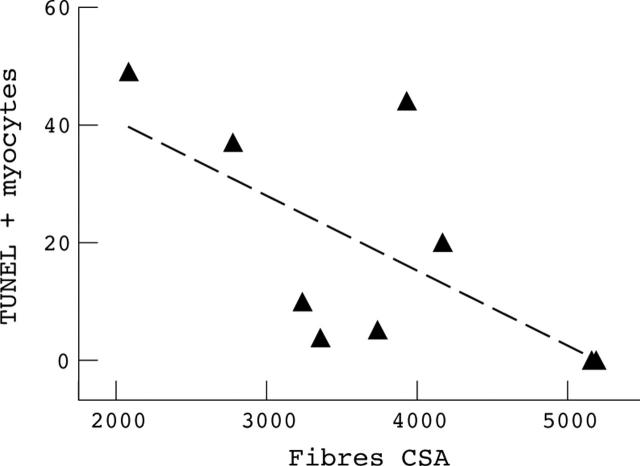
Figure 3 .
Linear regression between number of TUNEL positive myonuclei/mm3 and fibre cross sectional area (expressed as µm2) in the chronic heart failure patients (n = 9, p = 0.05). TUNEL, in situ nick end labelling.
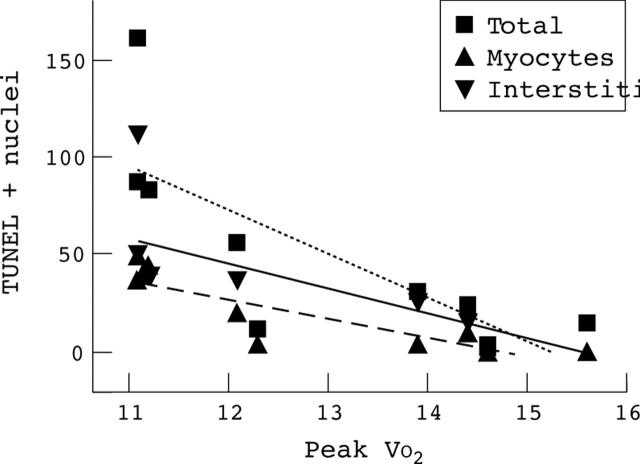
Figure 4 .
Linear regression between number of TUNEL positive nuclei/mm3 (total, myocyte, and interstitial) and peak VO2 in the chronic heart failure patients (ml/kg/min) (n = 9; p = 0.03 for total, p = 0.006 for myocyte, and p = 0.08 for interstitial cells).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams V., Jiang H., Yu J., Möbius-Winkler S., Fiehn E., Linke A., Weigl C., Schuler G., Hambrecht R. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999 Mar 15;33(4):959–965. doi: 10.1016/s0735-1097(98)00626-3. [DOI] [PubMed] [Google Scholar]
- Allen D. L., Linderman J. K., Roy R. R., Bigbee A. J., Grindeland R. E., Mukku V., Edgerton V. R. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997 Aug;273(2 Pt 1):C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- Anker S. D., Coats A. J. Cachexia in heart failure is bad for you. Eur Heart J. 1998 Feb;19(2):191–193. [PubMed] [Google Scholar]
- Anker S. D., Swan J. W., Volterrani M., Chua T. P., Clark A. L., Poole-Wilson P. A., Coats A. J. The influence of muscle mass, strength, fatigability and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. 1997 Feb;18(2):259–269. doi: 10.1093/oxfordjournals.eurheartj.a015229. [DOI] [PubMed] [Google Scholar]
- Arnolda L., Conway M., Dolecki M., Sharif H., Rajagopalan B., Ledingham J. G., Sleight P., Radda G. K. Skeletal muscle metabolism in heart failure: a 31P nuclear magnetic resonance spectroscopy study of leg muscle. Clin Sci (Lond) 1990 Dec;79(6):583–589. doi: 10.1042/cs0790583. [DOI] [PubMed] [Google Scholar]
- Carraro U., Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- Fink L. I., Wilson J. R., Ferraro N. Exercise ventilation and pulmonary artery wedge pressure in chronic stable congestive heart failure. Am J Cardiol. 1986 Feb 1;57(4):249–253. doi: 10.1016/0002-9149(86)90900-8. [DOI] [PubMed] [Google Scholar]
- Fleg J. L., Lakatta E. G. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol (1985) 1988 Sep;65(3):1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- Franciosa J. A., Park M., Levine T. B. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol. 1981 Jan;47(1):33–39. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- Harridge S. D., Magnusson G., Gordon A. Skeletal muscle contractile characteristics and fatigue resistance in patients with chronic heart failure. Eur Heart J. 1996 Jun;17(6):896–901. doi: 10.1093/oxfordjournals.eurheartj.a014971. [DOI] [PubMed] [Google Scholar]
- Harrington D., Anker S. D., Chua T. P., Webb-Peploe K. M., Ponikowski P. P., Poole-Wilson P. A., Coats A. J. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997 Dec;30(7):1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Libera L. D., Zennaro R., Sandri M., Ambrosio G. B., Vescovo G. Apoptosis and atrophy in rat slow skeletal muscles in chronic heart failure. Am J Physiol. 1999 Nov;277(5 Pt 1):C982–C986. doi: 10.1152/ajpcell.1999.277.5.C982. [DOI] [PubMed] [Google Scholar]
- Lipkin D. P., Jones D. A., Round J. M., Poole-Wilson P. A. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol. 1988 Feb;18(2):187–195. doi: 10.1016/0167-5273(88)90164-7. [DOI] [PubMed] [Google Scholar]
- Llovera M., García-Martínez C., Agell N., López-Soriano F. J., Argilés J. M. TNF can directly induce the expression of ubiquitin-dependent proteolytic system in rat soleus muscles. Biochem Biophys Res Commun. 1997 Jan 13;230(2):238–241. doi: 10.1006/bbrc.1996.5827. [DOI] [PubMed] [Google Scholar]
- Mancini D. M., Ferraro N., Tuchler M., Chance B., Wilson J. R. Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus-31 nuclear magnetic resonance. Am J Cardiol. 1988 Dec 1;62(17):1234–1240. doi: 10.1016/0002-9149(88)90266-4. [DOI] [PubMed] [Google Scholar]
- Mancini D. M., Walter G., Reichek N., Lenkinski R., McCully K. K., Mullen J. L., Wilson J. R. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992 Apr;85(4):1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- Massie B. M., Conway M., Rajagopalan B., Yonge R., Frostick S., Ledingham J., Sleight P., Radda G. Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure. Evidence for abnormalities unrelated to blood flow. Circulation. 1988 Aug;78(2):320–326. doi: 10.1161/01.cir.78.2.320. [DOI] [PubMed] [Google Scholar]
- Minotti J. R., Pillay P., Chang L., Wells L., Massie B. M. Neurophysiological assessment of skeletal muscle fatigue in patients with congestive heart failure. Circulation. 1992 Sep;86(3):903–908. doi: 10.1161/01.cir.86.3.903. [DOI] [PubMed] [Google Scholar]
- Narula J., Haider N., Virmani R., DiSalvo T. G., Kolodgie F. D., Hajjar R. J., Schmidt U., Semigran M. J., Dec G. W., Khaw B. A. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996 Oct 17;335(16):1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Abbi R., Quaini F., Kajstura J., Cheng W., Nitahara J. A., Quaini E., Di Loreto C., Beltrami C. A., Krajewski S. Apoptosis in the failing human heart. N Engl J Med. 1997 Apr 17;336(16):1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- Sandri M., Carraro U., Podhorska-Okolov M., Rizzi C., Arslan P., Monti D., Franceschi C. Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEBS Lett. 1995 Oct 16;373(3):291–295. doi: 10.1016/0014-5793(95)00908-r. [DOI] [PubMed] [Google Scholar]
- Sandri M., Podhorska-Okolow M., Geromel V., Rizzi C., Arslan P., Franceschi C., Carraro U. Exercise induces myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol. 1997 Jan;56(1):45–57. doi: 10.1097/00005072-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Shephard R. J., Bouhlel E., Vandewalle H., Monod H. Muscle mass as a factor limiting physical work. J Appl Physiol (1985) 1988 Apr;64(4):1472–1479. doi: 10.1152/jappl.1988.64.4.1472. [DOI] [PubMed] [Google Scholar]
- Simonini A., Long C. S., Dudley G. A., Yue P., McElhinny J., Massie B. M. Heart failure in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circ Res. 1996 Jul;79(1):128–136. doi: 10.1161/01.res.79.1.128. [DOI] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J., Green H. J., Cobb F. R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990 Feb;81(2):518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- Tews D. S., Goebel H. H., Schneider I., Gunkel A., Stennert E., Neiss W. F. DNA-fragmentation and expression of apoptosis-related proteins in experimentally denervated and reinnervated rat facial muscle. Neuropathol Appl Neurobiol. 1997 Apr;23(2):141–149. [PubMed] [Google Scholar]
- Vescovo G., Ceconi C., Bernocchi P., Ferrari R., Carraro U., Ambrosio G. B., Libera L. D. Skeletal muscle myosin heavy chain expression in rats with monocrotaline-induced cardiac hypertrophy and failure. Relation to blood flow and degree of muscle atrophy. Cardiovasc Res. 1998 Jul;39(1):233–241. doi: 10.1016/s0008-6363(98)00041-8. [DOI] [PubMed] [Google Scholar]
- Vescovo G., Dalla Libera L., Serafini F., Leprotti C., Facchin L., Volterrani M., Ceconi C., Ambrosio G. B. Improved exercise tolerance after losartan and enalapril in heart failure: correlation with changes in skeletal muscle myosin heavy chain composition. Circulation. 1998 Oct 27;98(17):1742–1749. doi: 10.1161/01.cir.98.17.1742. [DOI] [PubMed] [Google Scholar]
- Vescovo G., Serafini F., Dalla Libera L., Leprotti C., Facchin L., Tenderini P., Ambrosio G. B. Skeletal muscle myosin heavy chains in heart failure: correlation between magnitude of the isozyme shift, exercise capacity, and gas exchange measurements. Am Heart J. 1998 Jan;135(1):130–137. doi: 10.1016/s0002-8703(98)70353-9. [DOI] [PubMed] [Google Scholar]
- Vescovo G., Serafini F., Facchin L., Tenderini P., Carraro U., Dalla Libera L., Catani C., Ambrosio G. B. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart. 1996 Oct;76(4):337–343. doi: 10.1136/hrt.76.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo G., Zennaro R., Sandri M., Carraro U., Leprotti C., Ceconi C., Ambrosio G. B., Dalla Libera L. Apoptosis of skeletal muscle myofibers and interstitial cells in experimental heart failure. J Mol Cell Cardiol. 1998 Nov;30(11):2449–2459. doi: 10.1006/jmcc.1998.0807. [DOI] [PubMed] [Google Scholar]
- Volterrani M., Clark A. L., Ludman P. F., Swan J. W., Adamopoulos S., Piepoli M., Coats A. J. Predictors of exercise capacity in chronic heart failure. Eur Heart J. 1994 Jun;15(6):801–809. doi: 10.1093/oxfordjournals.eurheartj.a060588. [DOI] [PubMed] [Google Scholar]
- Wiener D. H., Fink L. I., Maris J., Jones R. A., Chance B., Wilson J. R. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation. 1986 Jun;73(6):1127–1136. doi: 10.1161/01.cir.73.6.1127. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Fink L., Maris J., Ferraro N., Power-Vanwart J., Eleff S., Chance B. Evaluation of energy metabolism in skeletal muscle of patients with heart failure with gated phosphorus-31 nuclear magnetic resonance. Circulation. 1985 Jan;71(1):57–62. doi: 10.1161/01.cir.71.1.57. [DOI] [PubMed] [Google Scholar]