Abstract
OBJECTIVE—To make a quantitative assessment of the relation between disarray, fibrosis, and small vessel disease in hypertrophic cardiomyopathy. DESIGN—Detailed macroscopic and histological examination at 19 segments of the left and right ventricle and the left atrial free wall. PATIENTS—72 patients with hypertrophic cardiomyopathy who had suffered sudden death or progression to end stage cardiac failure (resulting in death or heart transplantation). MAIN OUTCOME MEASURES—The presence of scarring, atrial dilatation, and a mitral valve impact lesion were noted, and heart weight, wall thickness, per cent disarray, per cent fibrosis, and per cent small vessel disease quantitated for each heart. RESULTS—Within an individual heart the magnitude of hypertrophy correlated with the severity of fibrosis (p = 0.006) and disarray (p = 0.0002). Overall, however, total heart weight related weakly but significantly to fibrosis (r = 0.4, p = 0.0001) and small vessel disease (r = 0.3, p = 0.03), but not to disarray. Disarray was greater in hearts with mild left ventricular hypertrophy (maximum wall thickness < 20 mm) and preserved systolic function (60.9 (26)% v 43 (20.4)% respectively, p = 0.02) and hearts without a mitral valve impact lesion (26.3% v 18.9%, p = 0.04), but was uninfluenced by sex. Fibrosis was influenced by sex (7% in male patients and 4% in female, p = 0.04), but not by the presence of an impact lesion. No relation was found between disarray, fibrosis, and small vessel disease. CONCLUSIONS—Myocyte disarray is probably a direct response to functional or structural abnormalities of the mutated sarcomeric protein, while fibrosis and small vessel disease are secondary phenomena unrelated to disarray, but modified by factors such as left ventricular mass, sex, and perhaps local autocrine factors. Keywords: hypertrophic cardiomyopathy; histopathology; small vessel disease
Full Text
The Full Text of this article is available as a PDF (187.2 KB).
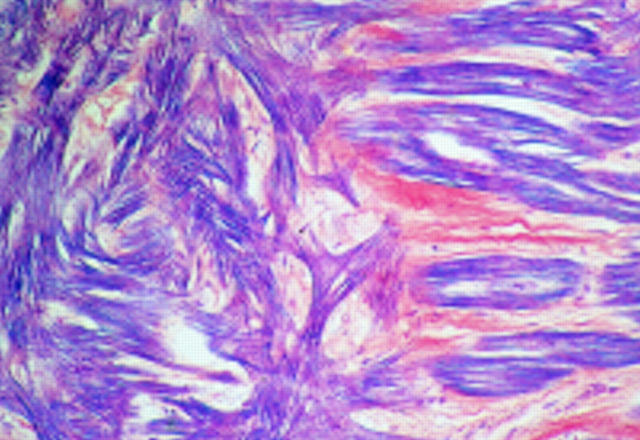
Figure 1 .
Section showing focal distribution of myocyte disarray (to the left) adjacent to normal parallel alignment of myocytes (×16 objective).
Figure 2 .
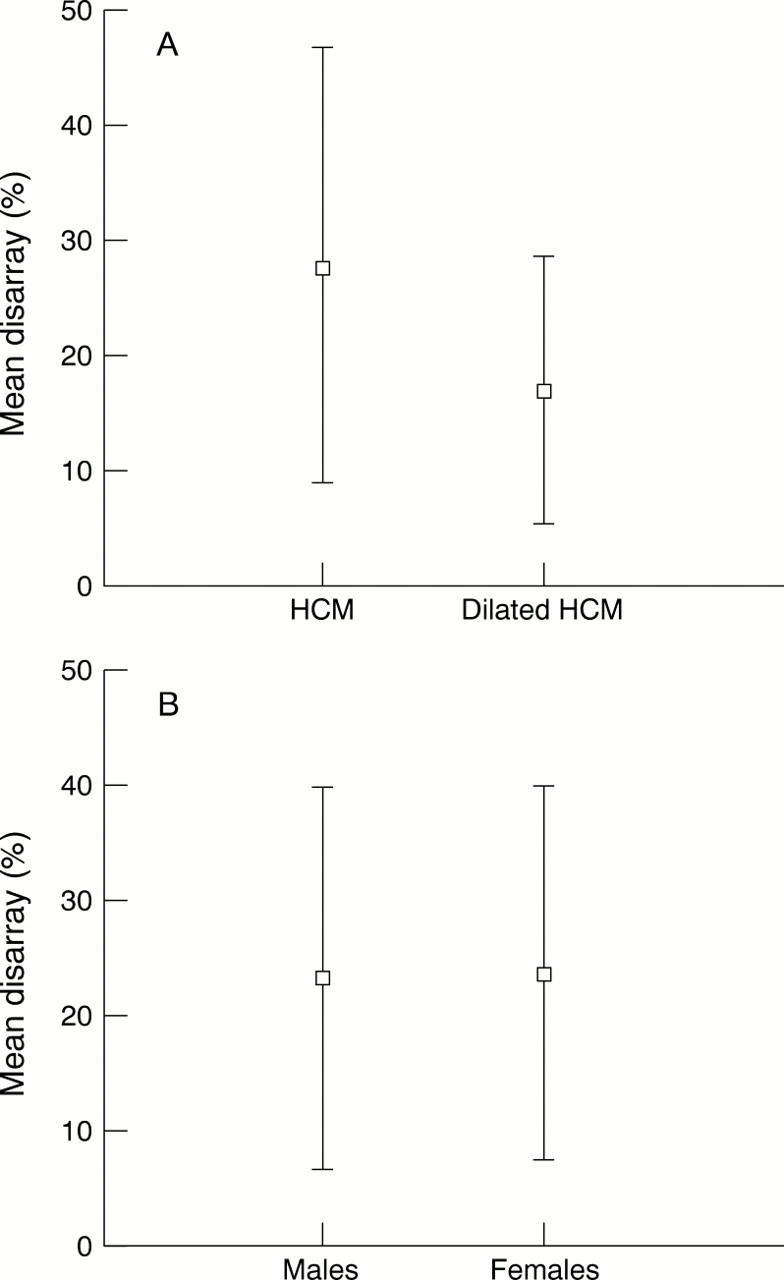
Disarray v disease status (A) and sex (B).
Figure 3 .
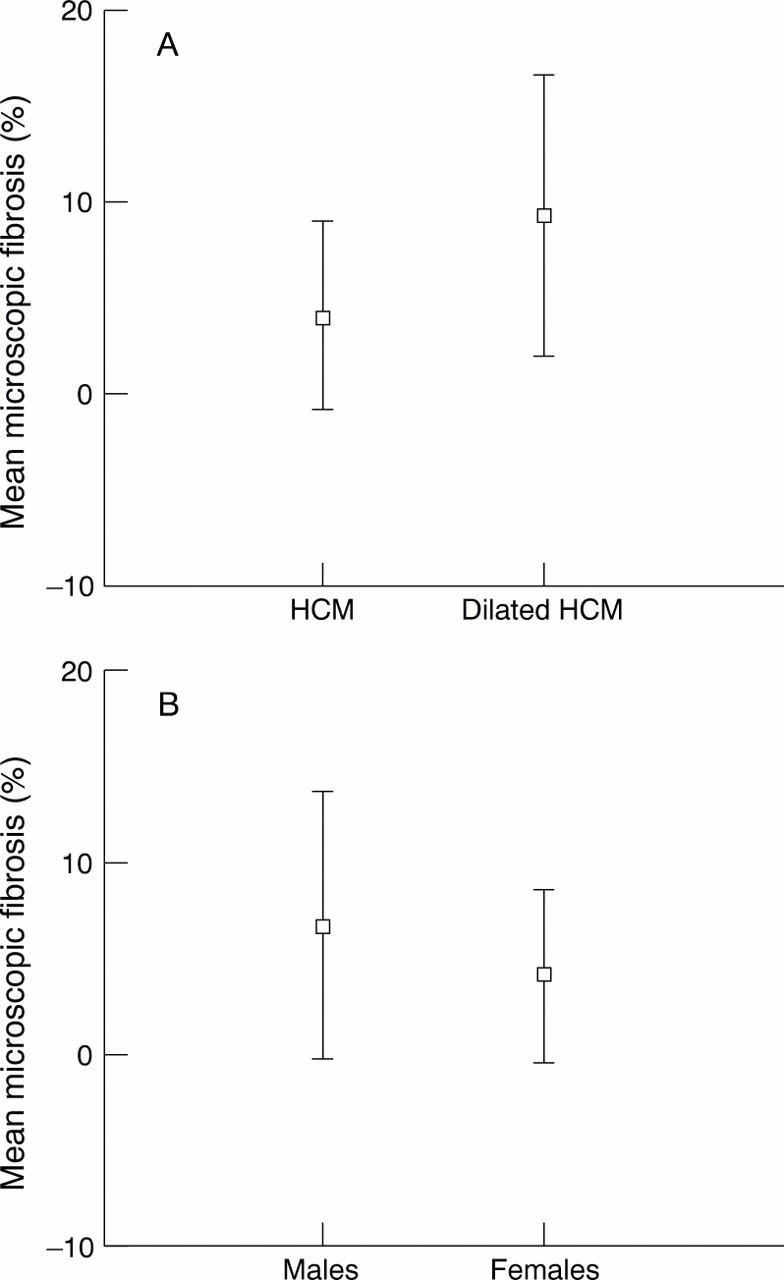
Microscopic fibrosis versus disease status (A) and sex (B).
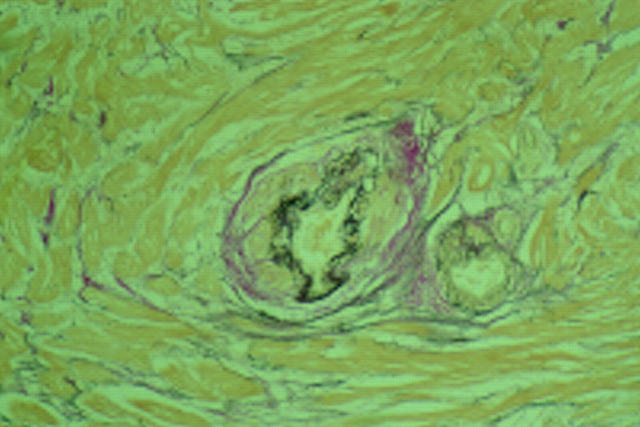
Figure 4 .
Dysplastic small vessel with no surrounding microscopic fibrosis (×10 objective).
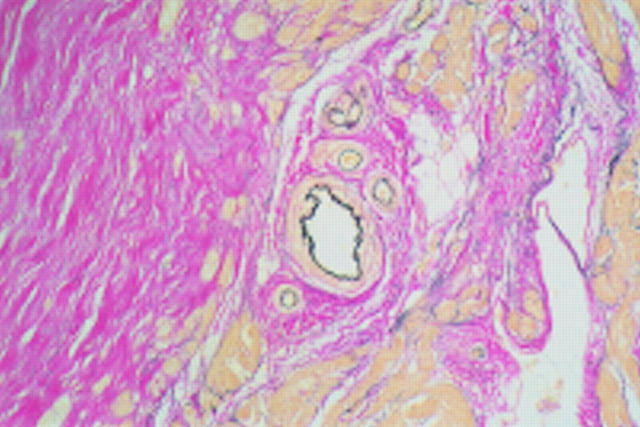
Figure 5 .
Pronounced fibrosis (sheets of collagen stained pink) with adjacent normal small vessel (×10 objective).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies M. J. The current status of myocardial disarray in hypertrophic cardiomyopathy. Br Heart J. 1984 Apr;51(4):361–363. doi: 10.1136/hrt.51.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisterfer-Lowrance A. A., Christe M., Conner D. A., Ingwall J. S., Schoen F. J., Seidman C. E., Seidman J. G. A mouse model of familial hypertrophic cardiomyopathy. Science. 1996 May 3;272(5262):731–734. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- Marian A. J., Roberts R. Recent advances in the molecular genetics of hypertrophic cardiomyopathy. Circulation. 1995 Sep 1;92(5):1336–1347. doi: 10.1161/01.cir.92.5.1336. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Ferrans V. J., Henry W. L., Clark C. E., Redwood D. R., Roberts W. C., Morrow A. G., Epstein S. E. Differences in distribution of myocardial abnormalities in patients with obstructive and nonobstructive asymmetric septal hypertrophy (ASH). Light and electron microscopic findings. Circulation. 1974 Sep;50(3):436–446. doi: 10.1161/01.cir.50.3.436. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Henry W. L., Roberts W. C., Epstein S. E. Comparison of echocardiographic and necropsy measurements of ventricular wall thicknesses in patients with and without disproportionate septal thickening. Circulation. 1977 Feb;55(2):341–346. doi: 10.1161/01.cir.55.2.341. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Kragel A. H., Roberts W. C. Sudden death in hypertrophic cardiomyopathy with normal left ventricular mass. Br Heart J. 1990 May;63(5):308–310. doi: 10.1136/hrt.63.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron B. J., Roberts W. C. Hypertrophic cardiomyopathy and cardiac muscle cell disorganization revisited: relation between the two and significance. Am Heart J. 1981 Jul;102(1):95–110. doi: 10.1016/0002-8703(81)90419-1. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Wolfson J. K., Epstein S. E., Roberts W. C. Intramural ("small vessel") coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986 Sep;8(3):545–557. doi: 10.1016/s0735-1097(86)80181-4. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Wolfson J. K., Epstein S. E., Roberts W. C. Morphologic evidence for "small vessel disease" in patients with hypertrophic cardiomyopathy. Z Kardiol. 1987;76 (Suppl 3):91–100. [PubMed] [Google Scholar]
- Maron B. J., Wolfson J. K., Roberts W. C. Relation between extent of cardiac muscle cell disorganization and left ventricular wall thickness in hypertrophic cardiomyopathy. Am J Cardiol. 1992 Sep 15;70(7):785–790. doi: 10.1016/0002-9149(92)90560-l. [DOI] [PubMed] [Google Scholar]
- Schwartz K., Carrier L., Guicheney P., Komajda M. Molecular basis of familial cardiomyopathies. Circulation. 1995 Jan 15;91(2):532–540. doi: 10.1161/01.cir.91.2.532. [DOI] [PubMed] [Google Scholar]
- Spirito P., Maron B. J. Relation between extent of left ventricular hypertrophy and age in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1989 Mar 15;13(4):820–823. doi: 10.1016/0735-1097(89)90222-2. [DOI] [PubMed] [Google Scholar]
- St John Sutton M. G., Lie J. T., Anderson K. R., O'Brien P. C., Frye R. L. Histopathological specificity of hypertrophic obstructive cardiomyopathy. Myocardial fibre disarray and myocardial fibrosis. Br Heart J. 1980 Oct;44(4):433–443. doi: 10.1136/hrt.44.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEARE D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J. 1958 Jan;20(1):1–8. doi: 10.1136/hrt.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Fujiwara H., Onodera T., Wu D. J., Matsuda M., Hamashima Y., Kawai C. Quantitative analysis of narrowings of intramyocardial small arteries in normal hearts, hypertensive hearts, and hearts with hypertrophic cardiomyopathy. Circulation. 1987 Jun;75(6):1130–1139. doi: 10.1161/01.cir.75.6.1130. [DOI] [PubMed] [Google Scholar]
- Watkins H. Genotype: phenotype correlations in hypertrophic cardiomyopathy. Eur Heart J. 1998 Jan;19(1):10–12. [PubMed] [Google Scholar]
- Yutani C., Imakita M., Ishibashi-Ueda H., Hatanaka K., Nagata S., Sakakibara H., Nimura Y. Three autopsy cases of progression to left ventricular dilatation in patients with hypertrophic cardiomyopathy. Am Heart J. 1985 Mar;109(3 Pt 1):545–553. doi: 10.1016/0002-8703(85)90561-7. [DOI] [PubMed] [Google Scholar]
- ten Cate F. J., Roelandt J. Progression to left ventricular dilatation in patients with hypertrophic obstructive cardiomyopathy. Am Heart J. 1979 Jun;97(6):762–765. doi: 10.1016/0002-8703(79)90012-7. [DOI] [PubMed] [Google Scholar]