Abstract
OBJECTIVE—To determine the outcome of heart transplantation for end stage amyloid heart disease in patients treated at a single centre. DESIGN—Records of all patients with amyloid heart disease who underwent heart transplantation were examined to determine survival, graft involvement by amyloid, the course of systemic amyloid disease, and the cause of death. PATIENTS—10 patients, mean (SD) age 54 (8) years, received transplants in the 13 year period 1984 to 1997. RESULTS—Two patients, both with AL amyloid (primary systemic amyloidosis), died perioperatively. Mean follow up in the remaining eight patients was 49.9 (39.5) months (range 3-116 months). Amyloid deposits in the grafts became evident histologically in five patients with AL amyloid at 5, 11, 12, 28, and 30 months after transplantation, and in one patient with familial amyloid at 60 months. Echocardiography showed no evidence of left ventricular systolic impairment at the time of recurrence. Seven patients died, at 3, 11, 26, 32, 49, 85, and 116 months after transplantation; four of these deaths were related to amyloidosis. Actuarial survival at one and two years was 60% and at five years, 30%. CONCLUSIONS—Heart transplantation for amyloid heart disease remains controversial because of the scarcity of hearts for transplantation, the systemic nature of amyloidosis, and the potential for amyloid deposition in the graft. Postoperative mortality was high (20%), reflecting extracardiac amyloid. Heart transplantation for end stage cardiac amyloidosis is feasible but, without treatment of the underlying process, it is a palliative procedure. Keywords: heart transplantation; amyloid heart disease; heart failure
Full Text
The Full Text of this article is available as a PDF (134.4 KB).
Figure 1 .
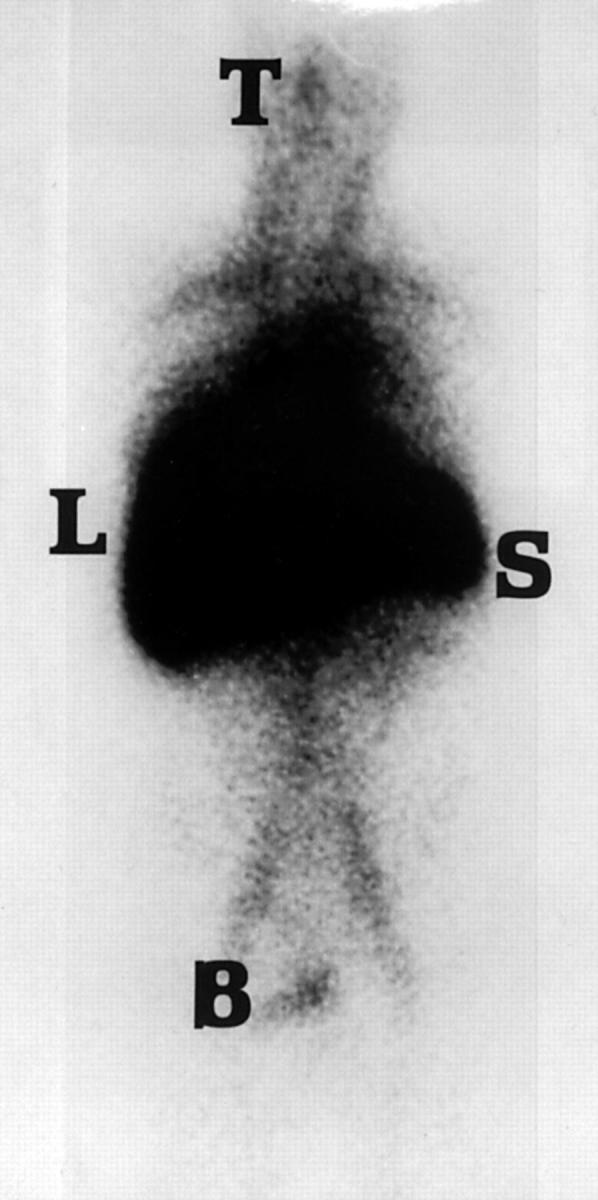
Anterior whole body 123I-SAP scan of patient 7 shortly after heart transplantation. Substantial amyloid is present in the liver and spleen which regressed to undetectable levels following chemotherapy. The remainder of the image represents tracer in the blood pool, the amount of which is inversely proportional to the whole body amyloid load. Tracer has accumulated in the thyroid and bladder. B, bladder; L, liver; S, spleen; T, thyroid.
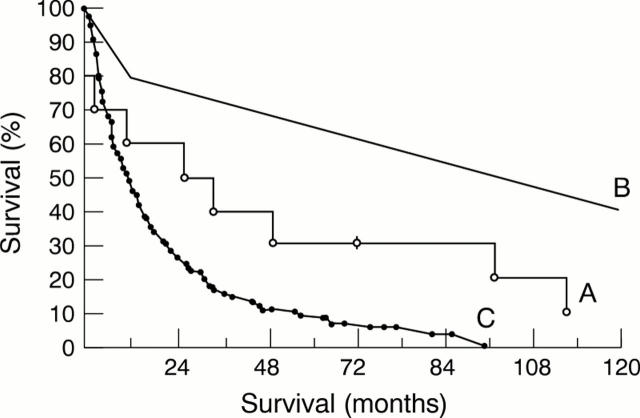
Figure 2 .
Actuarial survival of: (A) patients transplanted at Harefield Hospital for amyloid heart disease (n =10); (B) patients following heart transplantation for all causes (n = 32 579) (with the permission of the Journal of The International Society for Heart and Lung Transplantation18); (C) non-transplanted patients with heart failure from amyloid heart disease (n = 161) (with the permission of the Quarterly Journal of Medicine4).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson M. D. Inherited amyloidosis. J Med Genet. 1991 Feb;28(2):73–78. doi: 10.1136/jmg.28.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Liepnieks J., Uemichi T., Wheeler G., Correa R. Hereditary renal amyloidosis associated with a mutant fibrinogen alpha-chain. Nat Genet. 1993 Mar;3(3):252–255. doi: 10.1038/ng0393-252. [DOI] [PubMed] [Google Scholar]
- Billingham M. E., Cary N. R., Hammond M. E., Kemnitz J., Marboe C., McCallister H. A., Snovar D. C., Winters G. L., Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990 Nov-Dec;9(6):587–593. [PubMed] [Google Scholar]
- Comenzo R. L., Vosburgh E., Falk R. H., Sanchorawala V., Reisinger J., Dubrey S., Dember L. M., Berk J. L., Akpek G., LaValley M. Dose-intensive melphalan with blood stem-cell support for the treatment of AL (amyloid light-chain) amyloidosis: survival and responses in 25 patients. Blood. 1998 May 15;91(10):3662–3670. [PubMed] [Google Scholar]
- Comenzo R. L., Vosburgh E., Simms R. W., Bergethon P., Sarnacki D., Finn K., Dubrey S., Faller D. V., Wright D. G., Falk R. H. Dose-intensive melphalan with blood stem cell support for the treatment of AL amyloidosis: one-year follow-up in five patients. Blood. 1996 Oct 1;88(7):2801–2806. [PubMed] [Google Scholar]
- Conner R., Hosenpud J. D., Norman D. J., Pantely G. A., Cobanoglu A., Starr A. Heart transplantation for cardiac amyloidosis: successful one-year outcome despite recurrence of the disease. J Heart Transplant. 1988 Mar-Apr;7(2):165–167. [PubMed] [Google Scholar]
- Costanzo M. R., Augustine S., Bourge R., Bristow M., O'Connell J. B., Driscoll D., Rose E. Selection and treatment of candidates for heart transplantation. A statement for health professionals from the Committee on Heart Failure and Cardiac Transplantation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1995 Dec 15;92(12):3593–3612. doi: 10.1161/01.cir.92.12.3593. [DOI] [PubMed] [Google Scholar]
- Dubrey S., Simms R. W., Skinner M., Falk R. H. Recurrence of primary (AL) amyloidosis in a transplanted heart with four-year survival. Am J Cardiol. 1995 Oct 1;76(10):739–741. doi: 10.1016/s0002-9149(99)80214-8. [DOI] [PubMed] [Google Scholar]
- Falk R. H., Comenzo R. L., Skinner M. The systemic amyloidoses. N Engl J Med. 1997 Sep 25;337(13):898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- Gertz M. A., Kyle R. A., Greipp P. R. Response rates and survival in primary systemic amyloidosis. Blood. 1991 Jan 15;77(2):257–262. [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Hall R., Hawkins P. N. Cardiac transplantation for AL amyloidosis. BMJ. 1994 Oct 29;309(6962):1135–1137. doi: 10.1136/bmj.309.6962.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. N., Lavender J. P., Pepys M. B. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990 Aug 23;323(8):508–513. doi: 10.1056/NEJM199008233230803. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N., Pepys M. B. Imaging amyloidosis with radiolabelled SAP. Eur J Nucl Med. 1995 Jul;22(7):595–599. doi: 10.1007/BF01254559. [DOI] [PubMed] [Google Scholar]
- Holmgren G., Ericzon B. G., Groth C. G., Steen L., Suhr O., Andersen O., Wallin B. G., Seymour A., Richardson S., Hawkins P. N. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet. 1993 May 1;341(8853):1113–1116. doi: 10.1016/0140-6736(93)93127-m. [DOI] [PubMed] [Google Scholar]
- Hongo M., Ikeda S. Echocardiographic assessment of the evolution of amyloid heart disease: a study with familial amyloid polyneuropathy. Circulation. 1986 Feb;73(2):249–256. doi: 10.1161/01.cir.73.2.249. [DOI] [PubMed] [Google Scholar]
- Hosenpud J. D., Novick R. J., Bennett L. E., Keck B. M., Fiol B., Daily O. P. The Registry of the International Society for Heart and Lung Transplantation: thirteenth official report--1996. J Heart Lung Transplant. 1996 Jul;15(7):655–674. [PubMed] [Google Scholar]
- Hosenpud J. D., Uretsky B. F., Griffith B. P., O'Connell J. B., Olivari M. T., Valantine H. A. Successful intermediate-term outcome for patients with cardiac amyloidosis undergoing heart transplantation: results of a multicenter survey. J Heart Transplant. 1990 Jul-Aug;9(4):346–350. [PubMed] [Google Scholar]
- Kyle R. A., Gertz M. A. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995 Jan;32(1):45–59. [PubMed] [Google Scholar]
- Moreau P., Leblond V., Bourquelot P., Facon T., Huynh A., Caillot D., Hermine O., Attal M., Hamidou M., Nedellec G. Prognostic factors for survival and response after high-dose therapy and autologous stem cell transplantation in systemic AL amyloidosis: a report on 21 patients. Br J Haematol. 1998 Jun;101(4):766–769. doi: 10.1046/j.1365-2141.1998.00772.x. [DOI] [PubMed] [Google Scholar]
- Pelosi F., Jr, Capehart J., Roberts W. C. Effectiveness of cardiac transplantation for primary (AL) cardiac amyloidosis. Am J Cardiol. 1997 Feb 15;79(4):532–535. doi: 10.1016/s0002-9149(97)00806-0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Hawkins P. N., Booth D. R., Vigushin D. M., Tennent G. A., Soutar A. K., Totty N., Nguyen O., Blake C. C., Terry C. J. Human lysozyme gene mutations cause hereditary systemic amyloidosis. Nature. 1993 Apr 8;362(6420):553–557. doi: 10.1038/362553a0. [DOI] [PubMed] [Google Scholar]
- Soutar A. K., Hawkins P. N., Vigushin D. M., Tennent G. A., Booth S. E., Hutton T., Nguyen O., Totty N. F., Feest T. G., Hsuan J. J. Apolipoprotein AI mutation Arg-60 causes autosomal dominant amyloidosis. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7389–7393. doi: 10.1073/pnas.89.16.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangou A. J., Hawkins P. N., Heaton N. D., Rela M., Monaghan M., Nihoyannopoulos P., O'Grady J., Pepys M. B., Williams R. Progressive cardiac amyloidosis following liver transplantation for familial amyloid polyneuropathy: implications for amyloid fibrillogenesis. Transplantation. 1998 Jul 27;66(2):229–233. doi: 10.1097/00007890-199807270-00016. [DOI] [PubMed] [Google Scholar]
- Tan S. Y., Pepys M. B., Hawkins P. N. Treatment of amyloidosis. Am J Kidney Dis. 1995 Aug;26(2):267–285. doi: 10.1016/0272-6386(95)90647-9. [DOI] [PubMed] [Google Scholar]