Abstract
OBJECTIVE—To determine whether exercise is capable of protecting the myocardium from experimental infarction and to explore the involvement of protein kinase C, a key signalling protein, in the development of any protection observed. METHODS—Rats were exercised on a treadmill for 30 minutes at 23-27 m/min. Sham treated animals were placed on the stationary treadmill but not exercised. Twenty four hours later, hearts were Langendorff perfused and subjected to 35 minute left main coronary artery occlusion followed by 120 minute reperfusion. Infarct size was determined by tetrazolium staining and expressed as a percentage of the risk zone (I/R%). To examine the potential signalling pathway, animals were treated with either the selective protein kinase C inhibitor chelerythrine, 5 mg/kg intraperitoneally, or with vehicle 10 minutes before the exercise or sham treadmill period. RESULTS—In the non-exercised group, mean (SEM) I/R was 48.4 (3.0)%. In the exercised group, infarct size was reduced to 17.3 (3.0)% (p < 0.01). Infarct size limitation induced by exercise was abolished by chelerythrine (I/R 45.0 (6.0)%). Chelerythrine pretreatment alone did not have any effect on infarct size (I/R 51.1 (3.9)%). Differences in infarct size were independent of risk zone size and myocardial contractile function during ischaemia-reperfusion. CONCLUSIONS—Experimental moderate exercise induces protection against myocardial infarction 24 hours later. Protein kinase C activation during exercise appears to be an important signal mediator of this protective response. Keywords: exercise; myocardial infarction; cardioprotection; protein kinase C
Full Text
The Full Text of this article is available as a PDF (155.3 KB).
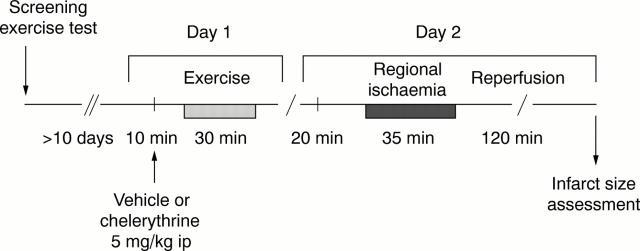
Figure 1 .
Experimental protocol. Rats were screened before the experiment for their ability to run on a treadmill. At least 10 days after screening, those animals that were able to run for 30 minutes were randomly assigned to either treadmill exercise or a corresponding sham period (day 1). Twenty four hours later (day 2), hearts were isolated and Langendorff perfused. They were subjected to 35 minutes of coronary artery occlusion and reperfusion, after which the infarct size was assessed. ip, intraperitoneally.
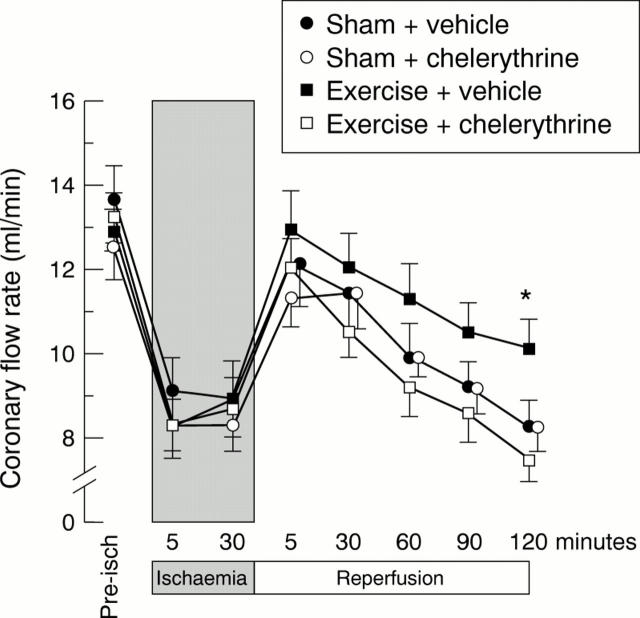
Figure 2 .
Changes in coronary flow during an in vitro (Langendorff perfusion) ischaemia-reperfusion protocol. Rats were treated with vehicle or chelerythrine chloride (5 mg/kg intraperitoneally) 10 minutes before exercise or sham treatment and 24 hours before ischaemia (see fig 1). *p < 0.05 v sham + vehicle group (n = 7/group).
Figure 3 .
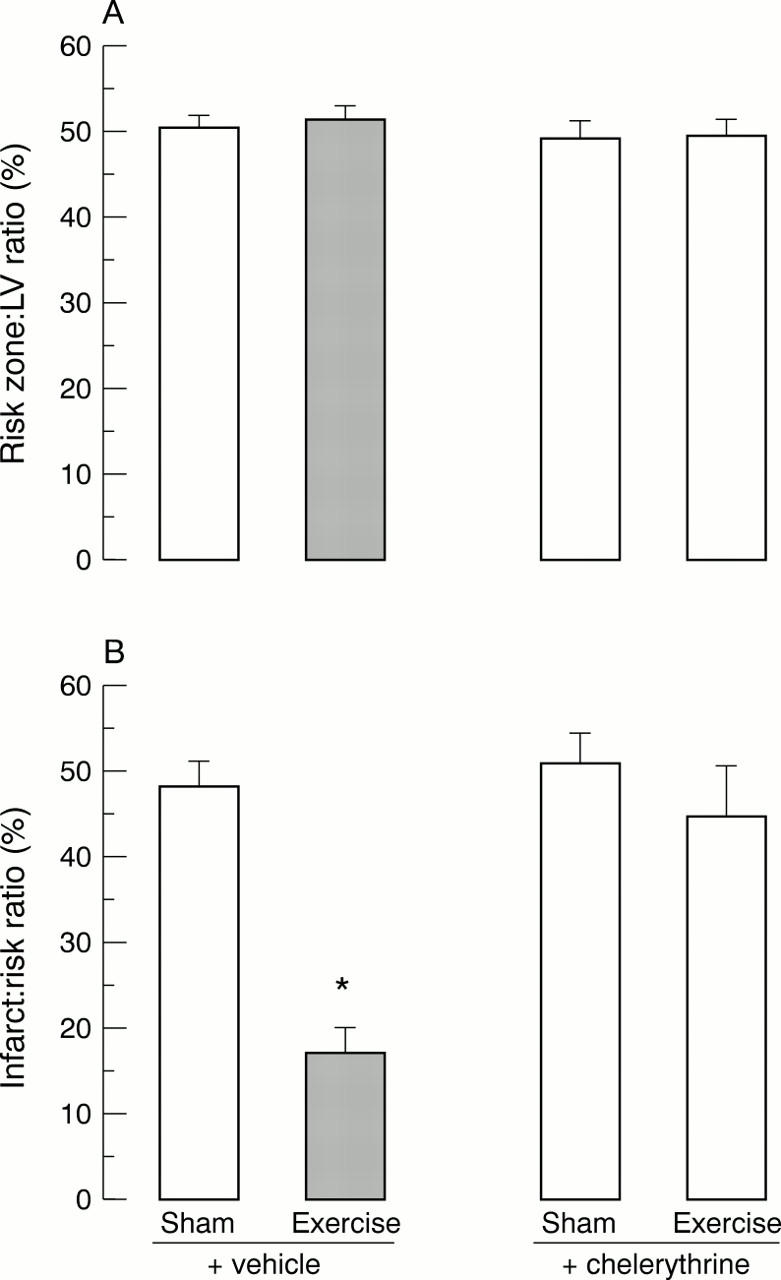
(A) Risk zone size expressed as percentage of total left ventricle muscle volume. (B) Infarct size expressed as percentage infarction of the risk zone in hearts subjected to 35 minutes of coronary occlusion followed by 120 minutes of reperfusion. Values are means, error bars = SEM. Rats were treated with vehicle or chelerythrine chloride (5 mg/kg intraperitoneally) 10 minutes before exercise or sham treatment and 24 hours before ischaemia (see fig 1). *p < 0.01 v sham + vehicle group (n = 7/group).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter G. F., Goma F. M., Yellon D. M. Involvement of protein kinase C in the delayed cytoprotection following sublethal ischaemia in rabbit myocardium. Br J Pharmacol. 1995 May;115(2):222–224. doi: 10.1111/j.1476-5381.1995.tb15866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter G. F., Marber M. S., Patel V. C., Yellon D. M. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994 Dec;90(6):2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- Baxter G. F., Mocanu M. M., Yellon D. M. Attenuation of myocardial ischaemic injury 24 h after diacylglycerol treatment in vivo. J Mol Cell Cardiol. 1997 Jul;29(7):1967–1975. doi: 10.1006/jmcc.1997.0436. [DOI] [PubMed] [Google Scholar]
- Bijnen F. C., Caspersen C. J., Mosterd W. L. Physical inactivity as a risk factor for coronary heart disease: a WHO and International Society and Federation of Cardiology position statement. Bull World Health Organ. 1994;72(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Bowles D. K., Farrar R. P., Starnes J. W. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am J Physiol. 1992 Sep;263(3 Pt 2):H804–H809. doi: 10.1152/ajpheart.1992.263.3.H804. [DOI] [PubMed] [Google Scholar]
- Bowles D. K., Starnes J. W. Exercise training improves metabolic response after ischemia in isolated working rat heart. J Appl Physiol (1985) 1994 Apr;76(4):1608–1614. doi: 10.1152/jappl.1994.76.4.1608. [DOI] [PubMed] [Google Scholar]
- Currie R. W., Karmazyn M., Kloc M., Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988 Sep;63(3):543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- Fishbein M. C., Meerbaum S., Rit J., Lando U., Kanmatsuse K., Mercier J. C., Corday E., Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981 May;101(5):593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- Fletcher G. F., Balady G., Froelicher V. F., Hartley L. H., Haskell W. L., Pollock M. L. Exercise standards. A statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1995 Jan 15;91(2):580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- Fletcher G. F., Blair S. N., Blumenthal J., Caspersen C., Chaitman B., Epstein S., Falls H., Froelicher E. S., Froelicher V. F., Pina I. L. Statement on exercise. Benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart association. Circulation. 1992 Jul;86(1):340–344. doi: 10.1161/01.cir.86.1.340. [DOI] [PubMed] [Google Scholar]
- Francis G. S., Goldsmith S. R., Ziesche S. M., Cohn J. N. Response of plasma norepinephrine and epinephrine to dynamic exercise in patients with congestive heart failure. Am J Cardiol. 1982 Apr 1;49(5):1152–1156. doi: 10.1016/0002-9149(82)90039-x. [DOI] [PubMed] [Google Scholar]
- Fryer R. M., Hsu A. K., Eells J. T., Nagase H., Gross G. J. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ Res. 1999 Apr 16;84(7):846–851. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Augereau J. M., Gleye J., Maffrand J. P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990 Nov 15;172(3):993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hutter M. M., Sievers R. E., Barbosa V., Wolfe C. L. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation. 1994 Jan;89(1):355–360. doi: 10.1161/01.cir.89.1.355. [DOI] [PubMed] [Google Scholar]
- Joyeux M., Baxter G. F., Thomas D. L., Ribuot C., Yellon D. M. Protein kinase C is involved in resistance to myocardial infarction induced by heat stress. J Mol Cell Cardiol. 1997 Dec;29(12):3311–3319. doi: 10.1006/jmcc.1997.0556. [DOI] [PubMed] [Google Scholar]
- Kaeffer N., Richard V., Thuillez C. Delayed coronary endothelial protection 24 hours after preconditioning: role of free radicals. Circulation. 1997 Oct 7;96(7):2311–2316. doi: 10.1161/01.cir.96.7.2311. [DOI] [PubMed] [Google Scholar]
- Koerner J. E., Terjung R. L. Effect of physical training on coronary collateral circulation of the rat. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):376–387. doi: 10.1152/jappl.1982.52.2.376. [DOI] [PubMed] [Google Scholar]
- Kuzuya T., Hoshida S., Yamashita N., Fuji H., Oe H., Hori M., Kamada T., Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993 Jun;72(6):1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- Laughlin M. H. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995 Aug;27(8):1135–1144. [PubMed] [Google Scholar]
- Locke M., Tanguay R. M., Klabunde R. E., Ianuzzo C. D. Enhanced postischemic myocardial recovery following exercise induction of HSP 72. Am J Physiol. 1995 Jul;269(1 Pt 2):H320–H325. doi: 10.1152/ajpheart.1995.269.1.H320. [DOI] [PubMed] [Google Scholar]
- Marber M. S., Latchman D. S., Walker J. M., Yellon D. M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993 Sep;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Maxwell M. P., Hearse D. J., Yellon D. M. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res. 1987 Oct;21(10):737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- Meng X., Shames B. D., Pulido E. J., Meldrum D. R., Ao L., Joo K. S., Harken A. H., Banerjee A. Adrenergic induction of bimodal myocardial protection: signal transduction and cardiac gene reprogramming. Am J Physiol. 1999 May;276(5 Pt 2):R1525–R1533. doi: 10.1152/ajpregu.1999.276.5.R1525. [DOI] [PubMed] [Google Scholar]
- Ping P., Zhang J., Qiu Y., Tang X. L., Manchikalapudi S., Cao X., Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997 Sep;81(3):404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Ping P., Tang X. L., Manchikalapudi S., Rizvi A., Zhang J., Takano H., Wu W. J., Teschner S., Bolli R. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that epsilon is the isoform involved. J Clin Invest. 1998 May 15;101(10):2182–2198. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schömig A., Dart A. M., Dietz R., Mayer E., Kübler W. Release of endogenous catecholamines in the ischemic myocardium of the rat. Part A: Locally mediated release. Circ Res. 1984 Nov;55(5):689–701. doi: 10.1161/01.res.55.5.689. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Pyne D. B. Exercise, training, and neutrophil function. Exerc Immunol Rev. 1997;3:96–116. [PubMed] [Google Scholar]
- Yamashita N., Hoshida S., Otsu K., Asahi M., Kuzuya T., Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999 Jun 7;189(11):1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N., Hoshida S., Taniguchi N., Kuzuya T., Hori M. Whole-body hyperthermia provides biphasic cardioprotection against ischemia/reperfusion injury in the rat. Circulation. 1998 Oct 6;98(14):1414–1421. doi: 10.1161/01.cir.98.14.1414. [DOI] [PubMed] [Google Scholar]