Abstract
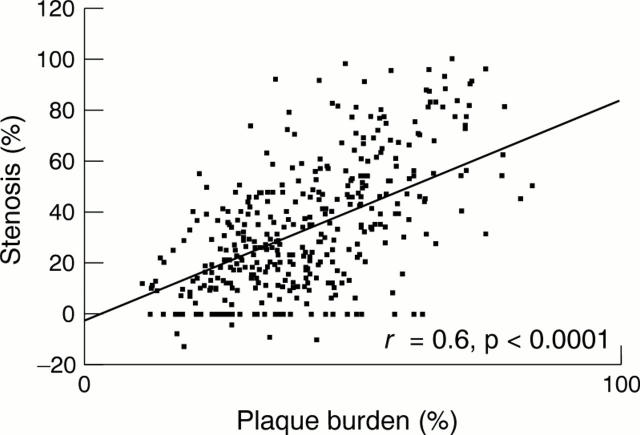
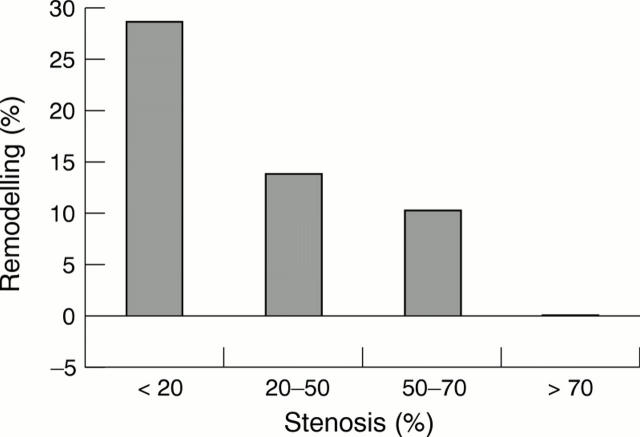
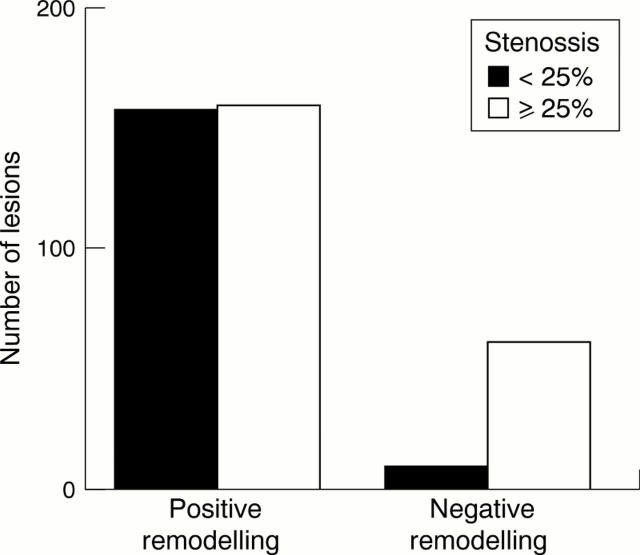
OBJECTIVES—To investigate the contribution of plaque size and vessel remodelling to coronary artery stenosis and to assess the role of vessel shrinkage (negative remodelling) across a wide range of lesions. DESIGN—Postmortem study of coronary remodelling in perfusion fixed hearts. SUBJECTS—24 men and 24 women who died suddenly with coronary artery disease. MAIN OUTCOME MEASURES—Percentage stenosis, percentage plaque burden, percentage remodelling, and arc of normal vessel were measured and related to age, sex, smoking status, and history of hypertension. RESULTS—There was a positive relation between percentage stenosis and percentage plaque burden (r = 0.6, p < 0.0001) and an inverse relation between percentage stenosis and percentage remodelling (r = -0.4, p < 0.0001). Multilinear regression modelling showed that luminal stenosis = 1.0 (plaque burden) − 0.4 (vessel remodelling). Remodelling was greater in lesions that would not have been significant at angiography (⩽ 25% stenosis) than in the remaining lesions (25.9 (26)% v 10.0 (21.1)%, p < 0.0001, respectively) and was reduced in segments with circumferential plaques (12.7 (24.5)% v 20.7 (24.3)% in eccentric plaques, p = 0.001). Remodelling did not correlate with age, sex, or smoking. Negative remodelling was present in 62 lesions with a stenosis > 25% versus 10 lesions with ⩽ 25% stenosis (p < 0.0001). Lesions with negative remodelling had greater plaque burden and luminal stenosis and a reduced arc of normal segment. CONCLUSION—Outward arterial remodelling negates the stenosing effect of increasing plaque size. Significant coronary stenoses arise from a failure of this outward remodelling in the face of a large plaque burden. Coronary arterial remodelling is unrelated to sex or smoking and is plaque specific. Keywords: coronary artery disease; vessel remodelling; pathology
Full Text
The Full Text of this article is available as a PDF (162.8 KB).
Figure 1 .
Luminal stenosis against plaque burden.
Figure 2 .
Luminal stenosis against vessel remodelling.
Figure 3 .
Number of lesions with positive versus negative remodelling according to percentage luminal stenosis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbruzzese T. A., Guzman R. J., Martin R. L., Yee C., Zarins C. K., Dalman R. L. Matrix metalloproteinase inhibition limits arterial enlargements in a rodent arteriovenous fistula model. Surgery. 1998 Aug;124(2):328–335. [PubMed] [Google Scholar]
- Bassiouny H. S., Song R. H., Hong X. F., Singh A., Kocharyan H., Glagov S. Flow regulation of 72-kD collagenase IV (MMP-2) after experimental arterial injury. Circulation. 1998 Jul 14;98(2):157–163. doi: 10.1161/01.cir.98.2.157. [DOI] [PubMed] [Google Scholar]
- DeBakey M. E., Lawrie G. M., Glaeser D. H. Patterns of atherosclerosis and their surgical significance. Ann Surg. 1985 Feb;201(2):115–131. doi: 10.1097/00000658-198502000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugelman M. Y., Virmani R., Correa R., Yu Z. X., Farb A., Leon M. B., Elami A., Fu Y. M., Casscells W., Epstein S. E. Smooth muscle cell abundance and fibroblast growth factors in coronary lesions of patients with nonfatal unstable angina. A clue to the mechanism of transformation from the stable to the unstable clinical state. Circulation. 1993 Dec;88(6):2493–2500. doi: 10.1161/01.cir.88.6.2493. [DOI] [PubMed] [Google Scholar]
- Glagov S., Weisenberg E., Zarins C. K., Stankunavicius R., Kolettis G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987 May 28;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- Hermiller J. B., Tenaglia A. N., Kisslo K. B., Phillips H. R., Bashore T. M., Stack R. S., Davidson C. J. In vivo validation of compensatory enlargement of atherosclerotic coronary arteries. Am J Cardiol. 1993 Mar 15;71(8):665–668. doi: 10.1016/0002-9149(93)91007-5. [DOI] [PubMed] [Google Scholar]
- Lerman A., Cannan C. R., Higano S. H., Nishimura R. A., Holmes D. R., Jr Coronary vascular remodeling in association with endothelial dysfunction. Am J Cardiol. 1998 May 1;81(9):1105–1109. doi: 10.1016/s0002-9149(98)00135-0. [DOI] [PubMed] [Google Scholar]
- Losordo D. W., Rosenfield K., Kaufman J., Pieczek A., Isner J. M. Focal compensatory enlargement of human arteries in response to progressive atherosclerosis. In vivo documentation using intravascular ultrasound. Circulation. 1994 Jun;89(6):2570–2577. doi: 10.1161/01.cir.89.6.2570. [DOI] [PubMed] [Google Scholar]
- Mann J., Davies M. J. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart. 1999 Sep;82(3):265–268. doi: 10.1136/hrt.82.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson D. D., Sirna S. J., Hiratzka L. F., Thorpe L., Armstrong M. L., Marcus M. L., Kerber R. E. Coronary arterial remodeling studied by high-frequency epicardial echocardiography: an early compensatory mechanism in patients with obstructive coronary atherosclerosis. J Am Coll Cardiol. 1991 Jan;17(1):79–86. doi: 10.1016/0735-1097(91)90707-g. [DOI] [PubMed] [Google Scholar]
- Mintz G. S., Kent K. M., Pichard A. D., Satler L. F., Popma J. J., Leon M. B. Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses. An intravascular ultrasound study. Circulation. 1997 Apr 1;95(7):1791–1798. doi: 10.1161/01.cir.95.7.1791. [DOI] [PubMed] [Google Scholar]
- Nishioka T., Luo H., Eigler N. L., Berglund H., Kim C. J., Siegel R. J. Contribution of inadequate compensatory enlargement to development of human coronary artery stenosis: an in vivo intravascular ultrasound study. J Am Coll Cardiol. 1996 Jun;27(7):1571–1576. doi: 10.1016/0735-1097(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G., Borst C., Post M. J., Mali W. P., Wensing P. J., Gussenhoven E. J., Hillen B. Atherosclerotic arterial remodeling in the superficial femoral artery. Individual variation in local compensatory enlargement response. Circulation. 1996 May 15;93(10):1818–1825. doi: 10.1161/01.cir.93.10.1818. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G., Wensing P. J., Post M. J., Hillen B., Mali W. P., Borst C. Paradoxical arterial wall shrinkage may contribute to luminal narrowing of human atherosclerotic femoral arteries. Circulation. 1995 Mar 1;91(5):1444–1449. doi: 10.1161/01.cir.91.5.1444. [DOI] [PubMed] [Google Scholar]
- Smits P. C., Bos L., Quarles van Ufford M. A., Eefting F. D., Pasterkamp G., Borst C. Shrinkage of human coronary arteries is an important determinant of de novo atherosclerotic luminal stenosis: an in vivo intravascular ultrasound study. Heart. 1998 Feb;79(2):143–147. doi: 10.1136/hrt.79.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronc F., Wassef M., Esposito B., Henrion D., Glagov S., Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996 Oct;16(10):1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- Varnava A. Coronary artery remodelling. Heart. 1998 Feb;79(2):109–110. doi: 10.1136/hrt.79.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. B., Porter T. R., Xie F., Deligonul U. Segmental analysis of coronary arteries with equivalent plaque burden by intravascular ultrasound in patients with and without angiographically significant coronary artery disease. Am J Cardiol. 1995 Sep 15;76(8):598–601. doi: 10.1016/s0002-9149(99)80163-5. [DOI] [PubMed] [Google Scholar]
- von Birgelen C., Mintz G. S., de Vrey E. A., Kimura T., Popma J. J., Airiian S. G., Leon M. B., Nobuyoshi M., Serruys P. W., de Feyter P. J. Atherosclerotic coronary lesions with inadequate compensatory enlargement have smaller plaque and vessel volumes: observations with three dimensional intravascular ultrasound in vivo. Heart. 1998 Feb;79(2):137–142. doi: 10.1136/hrt.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]