Abstract
OBJECTIVE—To test the potential of gene transfer approaches to enhance cardiac chronotropy in a porcine system as a model of the human heart. METHODS—Plasmids encoding either the human β2 adrenergic receptor or control constructs were injected into the right atria of native Yorkshire pig hearts. Percutaneous electrophysiological recording catheters equipped with 33 gauge circular injection needles were positioned in the mid-lateral right atrium. At the site of the earliest atrial potential the circular injection needles were rotated into the myocardium and the β2 adrenergic receptor (n = 6) or control plasmid constructs (n = 5) were injected. RESULTS—Injection of the β2 adrenergic receptor construct significantly enhanced chronotropy compared with control injections. The average (SD) heart rate of the pigs was 108 (16) beats/min before injection. Two days after injection with control plasmids the heart rate was 127 (25) beats/min (NS compared with preinjection rates). After injection with plasmid encoding the β2 adrenergic receptor the heart rate increased by 50% to 163 (33) beats/min (p < 0.05 compared with preinjection and postinjection control rates). CONCLUSIONS—The present studies showed in a large animal model that local targeting of gene expression may be a feasible modality to regulate cardiac pacemaking activity. In addition, these investigations provide an experimental basis for developing future clinical gene transfer approaches to upregulate heart rate and modulate cardiac conduction. Keywords: sinus node; adrenergic receptor; chronotropic agents; conduction system; gene therapy
Full Text
The Full Text of this article is available as a PDF (143.5 KB).
Figure 1 .
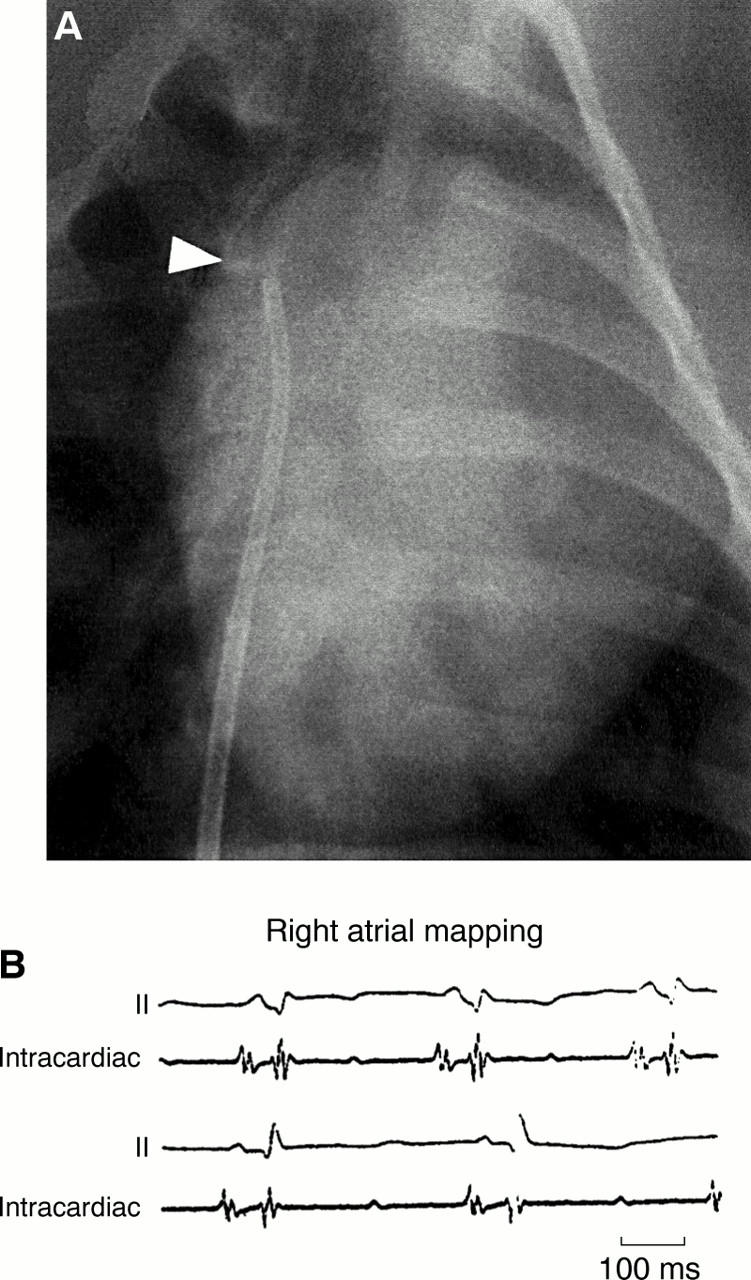
Cine of electrophysiological recording and injection catheter (arrow head) during injection of complementary DNA in to the porcine lateral right atrium (A). Two examples are shown of surface and intracardiac ECGs recorded before injection into the right atrium (B).
Figure 2 .
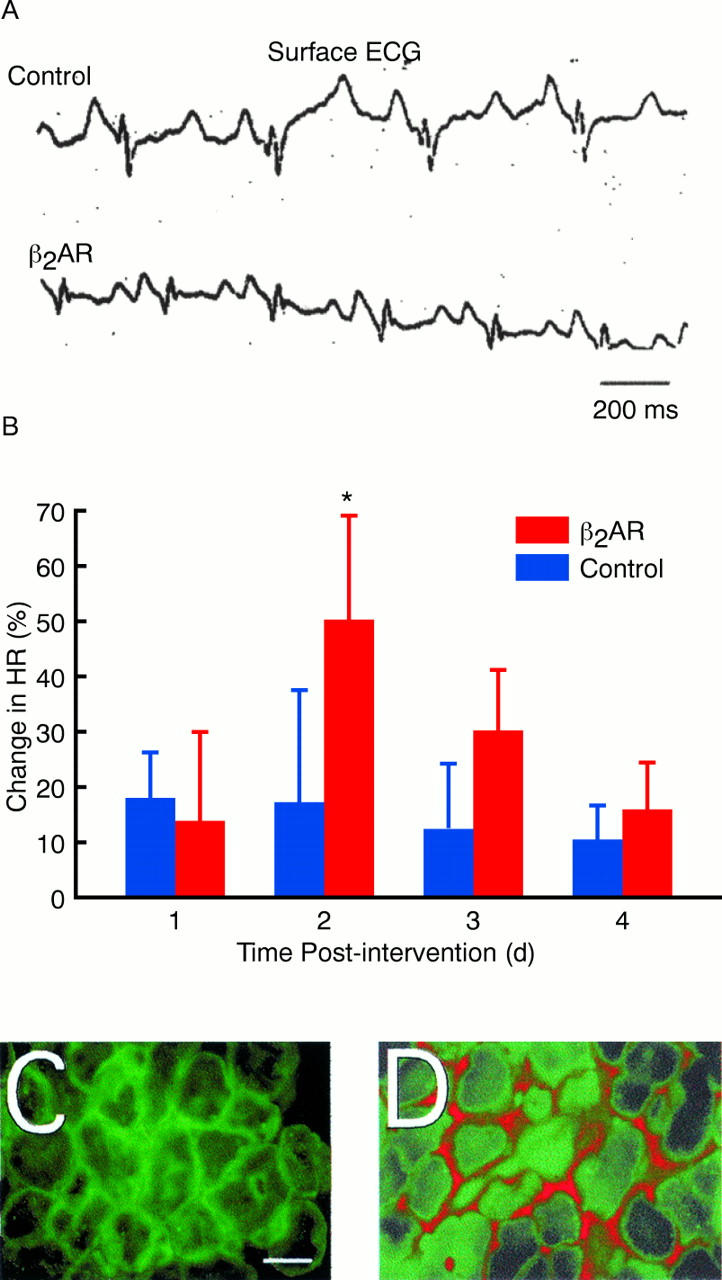
(A) Representative surface ECGs recorded 48 hours after injection of either control constructs or constructs encoding β2 adrenergic receptor. (B) The average percentage change in heart rate (HR) after injection of control construct or β2 adrenergic receptor (β2 AR) encoding plasmids (*p < 0.01 versus control). Dual fluorescence micrographs of sections of right atrial tissue co-injected with plasmids encoding green fluorescent protein and control constructs (C) and with the human β2 adrenergic receptor (D). Green fluorescent protein was visualised directly (green), and the human β2 adrenergic receptor was detected by immunostaining (red). Bar 32 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alagona P., Jr Advances in pacing for the patient with sick sinus syndrome. Curr Opin Cardiol. 1997 Jan;12(1):3–11. doi: 10.1097/00001573-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Alboni P., Menozzi C., Brignole M., Paparella N., Gaggioli G., Lolli G., Cappato R. Effects of permanent pacemaker and oral theophylline in sick sinus syndrome the THEOPACE study: a randomized controlled trial. Circulation. 1997 Jul 1;96(1):260–266. doi: 10.1161/01.cir.96.1.260. [DOI] [PubMed] [Google Scholar]
- Aoki M., Morishita R., Higaki J., Moriguchi A., Hayashi S., Matsushita H., Kida I., Tomita N., Sawa Y., Kaneda Y. Survival of grafts of genetically modified cardiac myocytes transfected with FITC-labeled oligodeoxynucleotides and the beta-galactosidase gene in the noninfarcted area, but not the myocardial infarcted area. Gene Ther. 1997 Feb;4(2):120–127. doi: 10.1038/sj.gt.3300361. [DOI] [PubMed] [Google Scholar]
- Asseman P., Berzin B., Desry D., Bauchart J. J., Reade R., Leroy O., Poncelet P., Lekieffre J., Thery C. Postextrasystolic sinoatrial exit block in human sick sinus syndrome: demonstration by direct recording of sinus node electrograms. Am Heart J. 1991 Dec;122(6):1633–1643. doi: 10.1016/0002-8703(91)90281-l. [DOI] [PubMed] [Google Scholar]
- Bharati S., Lev M. The pathologic changes in the conduction system beyond the age of ninety. Am Heart J. 1992 Aug;124(2):486–496. doi: 10.1016/0002-8703(92)90615-3. [DOI] [PubMed] [Google Scholar]
- Brignole M., Menozzi C., Lolli G., Oddone D., Gianfranchi L., Bertulla A. Pacing for carotid sinus syndrome and sick sinus syndrome. Pacing Clin Electrophysiol. 1990 Dec;13(12 Pt 2):2071–2075. doi: 10.1111/j.1540-8159.1990.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Buttrick P. M., Kass A., Kitsis R. N., Kaplan M. L., Leinwand L. A. Behavior of genes directly injected into the rat heart in vivo. Circ Res. 1992 Jan;70(1):193–198. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- Edelberg J. M., Aird W. C., Rosenberg R. D. Enhancement of murine cardiac chronotropy by the molecular transfer of the human beta2 adrenergic receptor cDNA. J Clin Invest. 1998 Jan 15;101(2):337–343. doi: 10.1172/JCI1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Epstein S. E. Gene therapy for vascular disease. FASEB J. 1995 Jul;9(10):843–851. doi: 10.1096/fasebj.9.10.7615154. [DOI] [PubMed] [Google Scholar]
- Gepstein L., Hayam G., Ben-Haim S. A. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation. 1997 Mar 18;95(6):1611–1622. doi: 10.1161/01.cir.95.6.1611. [DOI] [PubMed] [Google Scholar]
- Haas J., Park E. C., Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996 Mar 1;6(3):315–324. doi: 10.1016/s0960-9822(02)00482-7. [DOI] [PubMed] [Google Scholar]
- Heerdt P. M., Gandhi C. D., Dickstein M. L. Disparity of isoflurane effects on left and right ventricular afterload and hydraulic power generation in swine. Anesth Analg. 1998 Sep;87(3):511–521. doi: 10.1097/00000539-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Hughes H. C. Swine in cardiovascular research. Lab Anim Sci. 1986 Aug;36(4):348–350. [PubMed] [Google Scholar]
- Johns D. C., Nuss H. B., Chiamvimonvat N., Ramza B. M., Marban E., Lawrence J. H. Adenovirus-mediated expression of a voltage-gated potassium channel in vitro (rat cardiac myocytes) and in vivo (rat liver). A novel strategy for modifying excitability. J Clin Invest. 1995 Aug;96(2):1152–1158. doi: 10.1172/JCI118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira Júnior L. F., Beraldo P. S., Chapadeiro E., Jesus P. C. Cardiac autonomic dysfunction and neuroganglionitis in a rat model of chronic Chagas' disease. Cardiovasc Res. 1992 Apr;26(4):324–329. doi: 10.1093/cvr/26.4.324. [DOI] [PubMed] [Google Scholar]
- Kass-Eisler A., Falck-Pedersen E., Alvira M., Rivera J., Buttrick P. M., Wittenberg B. A., Cipriani L., Leinwand L. A. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R., Estioko M., Wiener I. Primary sick sinus syndrome as an indication for chronic pacemaker therapy in young adults: incidence, clinical features, and long-term evaluation. Am Heart J. 1982 Mar;103(3):338–342. doi: 10.1016/0002-8703(82)90271-x. [DOI] [PubMed] [Google Scholar]
- Koh G. Y., Soonpaa M. H., Klug M. G., Pride H. P., Cooper B. J., Zipes D. P., Field L. J. Stable fetal cardiomyocyte grafts in the hearts of dystrophic mice and dogs. J Clin Invest. 1995 Oct;96(4):2034–2042. doi: 10.1172/JCI118251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Laks H., Drinkwater D. C., Blitz A., Lam L., Shiraishi Y., Chang P., Drake T. A., Ardehali A. Cardiac gene transfer by intracoronary infusion of adenovirus vector-mediated reporter gene in the transplanted mouse heart. J Thorac Cardiovasc Surg. 1996 Jan;111(1):246–252. doi: 10.1016/S0022-5223(96)70422-1. [DOI] [PubMed] [Google Scholar]
- Libby P. Gene therapy of restenosis: promise and perils. Circ Res. 1998 Feb 23;82(3):404–406. doi: 10.1161/01.res.82.3.404. [DOI] [PubMed] [Google Scholar]
- Lin H., Parmacek M. S., Morle G., Bolling S., Leiden J. M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990 Dec;82(6):2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- Mehta A. V., Chidambaram B., Garrett A. Familial symptomatic sinus bradycardia: autosomal dominant inheritance. Pediatr Cardiol. 1995 Sep-Oct;16(5):231–234. doi: 10.1007/BF00795713. [DOI] [PubMed] [Google Scholar]
- Molina H. A., Milei J., Rimoldi M. T., Gonzalez Cappa S. M., Storino R. A. Histopathology of the heart conducting system in experimental Chagas disease in mice. Trans R Soc Trop Med Hyg. 1988;82(2):241–246. doi: 10.1016/0035-9203(88)90432-4. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Inoue T., Miwa S., Fujimoto T., Ohnishi Y., Fukuzaki H. Heart rate responses to autonomic drugs in sick sinus syndrome--correlation with syncope and electrophysiologic data. Jpn Circ J. 1991 Jan;55(1):15–23. doi: 10.1253/jcj.55.15. [DOI] [PubMed] [Google Scholar]
- Olofsson B. O., Eriksson P., Eriksson A. The sick sinus syndrome in familial amyloidosis with polyneuropathy. Int J Cardiol. 1983 Aug;4(1):71–73. doi: 10.1016/0167-5273(83)90217-6. [DOI] [PubMed] [Google Scholar]
- Onat A. Familial sinus node disease and degenerative myopia--a new hereditary syndrome? Hum Genet. 1986 Feb;72(2):182–184. doi: 10.1007/BF00283944. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. D., Schocken D. D. Update on sick sinus syndrome, a cardiac disorder of aging. Geriatrics. 1990 Jan;45(1):26-30, 33-6. [PubMed] [Google Scholar]
- Shaw D. B., Linker N. J., Heaver P. A., Evans R. Chronic sinoatrial disorder (sick sinus syndrome): a possible result of cardiac ischaemia. Br Heart J. 1987 Dec;58(6):598–607. doi: 10.1136/hrt.58.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi I., Takamatsu T., Minamikawa T., Onouchi Z., Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation. 1992 Jun;85(6):2176–2184. doi: 10.1161/01.cir.85.6.2176. [DOI] [PubMed] [Google Scholar]
- Sundeman H., Haney M., Broomé M., Häggmark S., Johansson G., Winsö O., Biber B. The effects of desflurane on cardiac function as measured by conductance volumetry in swine. Anesth Analg. 1998 Sep;87(3):522–528. doi: 10.1097/00000539-199809000-00003. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., Cederdahl J. E., Bers D. M. Quantitative analysis of regional variability in the distribution of transverse tubules in rabbit myocardium. Cell Tissue Res. 1991 May;264(2):293–298. doi: 10.1007/BF00313966. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Dowty M. E., Jiao S., Repetto G., Berg R. K., Ludtke J. J., Williams P., Slautterback D. B. Expression of naked plasmids by cultured myotubes and entry of plasmids into T tubules and caveolae of mammalian skeletal muscle. J Cell Sci. 1992 Dec;103(Pt 4):1249–1259. doi: 10.1242/jcs.103.4.1249. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Williams P., Acsadi G., Jiao S., Jani A., Chong W. Conditions affecting direct gene transfer into rodent muscle in vivo. Biotechniques. 1991 Oct;11(4):474–485. [PubMed] [Google Scholar]
- Wu D. L., Yeh S. J., Lin F. C., Wang C. C., Cherng W. J. Sinus automaticity and sinoatrial conduction in severe symptomatic sick sinus syndrome. J Am Coll Cardiol. 1992 Feb;19(2):355–364. doi: 10.1016/0735-1097(92)90492-6. [DOI] [PubMed] [Google Scholar]
- Yabek S. M., Dillon T., Berman W., Jr, Niland C. J. Symptomatic sinus node dysfunction in children without structural heart disease. Pediatrics. 1982 May;69(5):590–593. [PubMed] [Google Scholar]
- von Harsdorf R., Schott R. J., Shen Y. T., Vatner S. F., Mahdavi V., Nadal-Ginard B. Gene injection into canine myocardium as a useful model for studying gene expression in the heart of large mammals. Circ Res. 1993 Mar;72(3):688–695. doi: 10.1161/01.res.72.3.688. [DOI] [PubMed] [Google Scholar]


