Abstract
OBJECTIVE—To determine whether corrodible materials may be safely used as biodegradable cardiovascular implants. DESIGN—Corrodible iron stents (> 99.8% iron) were produced from pure iron and laser cut with a stent design similar to a commercially available permanent stent (PUVA-AS16). A total of 16 NOR-I stents were implanted into the native descending aorta of 16 New Zealand white rabbits (mean luminal diameter at the implantation site 3.4 mm, balloon diameter to vessel diameter ratio 1.13). RESULTS—No thromboembolic complications and no adverse events occurred during the follow up of 6-18 months. All stents were patent at repeat angiography after 6 (n = 9), 12 (n = 5), and 18 months (n = 2) with no significant neointimal proliferation, no pronounced inflammatory response, and no systemic toxicity. CONCLUSIONS—This initial in vivo experience suggests that degradable iron stents can be safely implanted without significant obstruction of the stented vessel caused by inflammation, neointimal proliferation, or thrombotic events. Keywords: congenital heart disease; corrosion; stents; biodegradation
Full Text
The Full Text of this article is available as a PDF (263.6 KB).
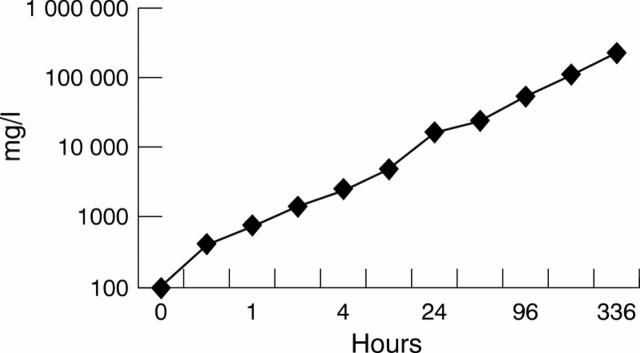
Figure 1 .
Accumulation of iron in the electrolyte in relation to time.
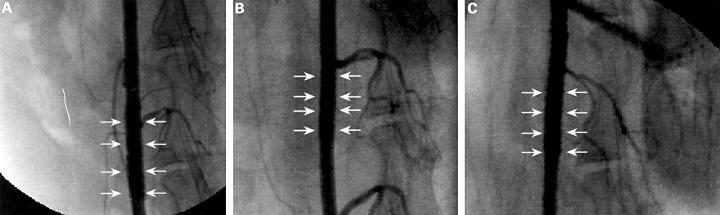
Figure 2 .
Lateral angiography of the stented descending aorta (A) six months, (B) 12 months, and (C) 18 months after implantation. There is complete patency of the vessel. (Arrows indicate stent implantation site.)
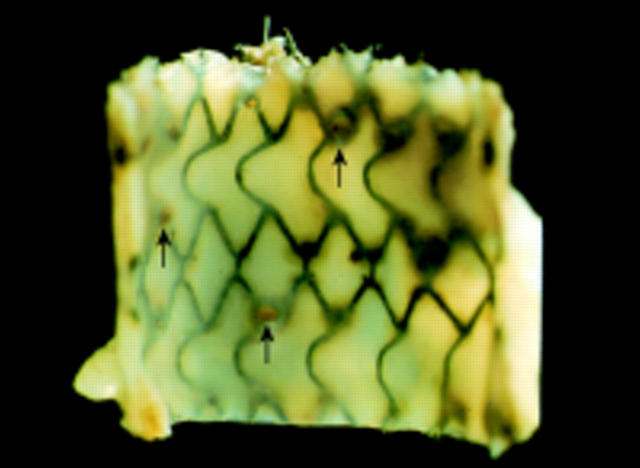
Figure 3 .
Rabbit aorta with degradable iron stent 12 months after implantation with numerous pinpoint, slightly raised plaques consisting of corroded stent material (arrows).
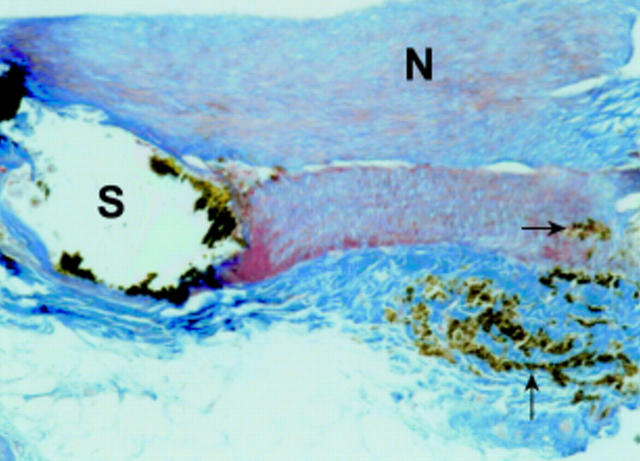
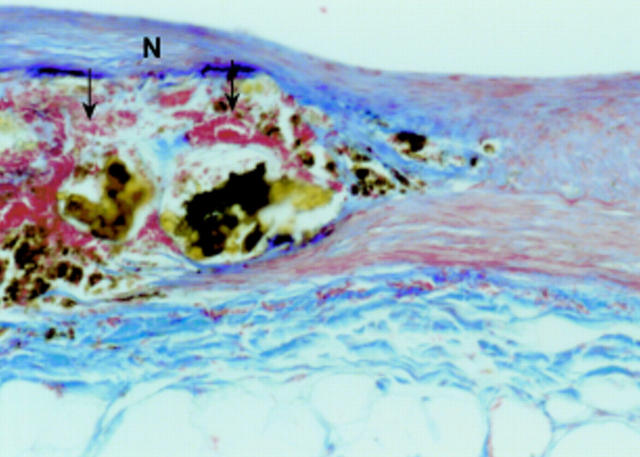
Figure 4 .
Rabbit aorta with degradable iron stent (S) six months after implantation; most of the stent struts are artificially lost and remnants of the iron are visible as plaques of brownish pigment. The stent strut is completely covered by neointima (N). Adjacent to the stent strut there is accumulation of iron laden macrophages within the media and adventitia (arrow). Azan stain, original magnification ×10.
Figure 5 .
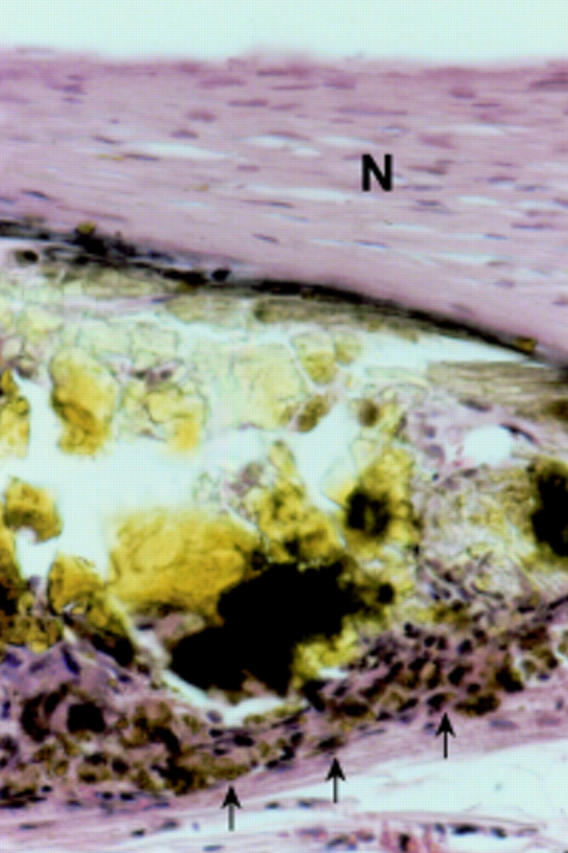
Rabbit aorta with degradable iron stent 18 months after implantation. A stent strut is covered by neointima (N); along the adventitial side there is moderate infiltration of macrophages (arrows). Haematoxylin and eosin stain, original magnification ×40.
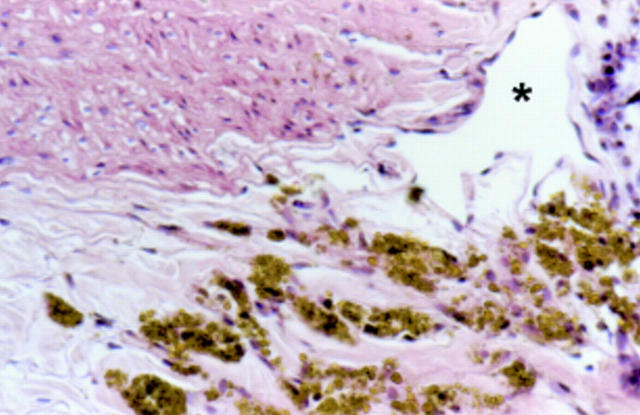
Figure 6 .
Rabbit aorta 18 months after iron stent implantation. Numerous siderophages are seen in the adventitia adjacent to a lymphatic capillary (*). Haematoxylin and eosin stain, original magnification ×40.
Figure 7 .
Rabbit aorta with iron stent 12 months after implantation. A stent strut is covered by neointima (N) and adjacent to the advanced degraded iron strut there is vascularisation with some capillaries (arrows). Azan stain, original magnification ×20.
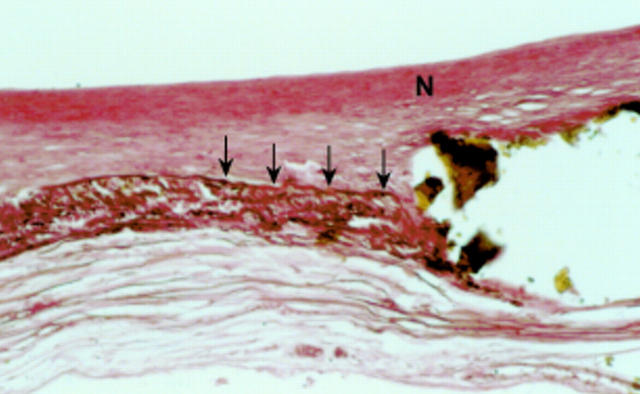
Figure 8 .
Rabbit aorta with degradable iron stent 18 months after implantation showing a stent strut covered by neointima (N); media and internal elastic membrane destroyed adjacent to the stent strut. Elastic stain; original magnification ×20.
Figure 9 .
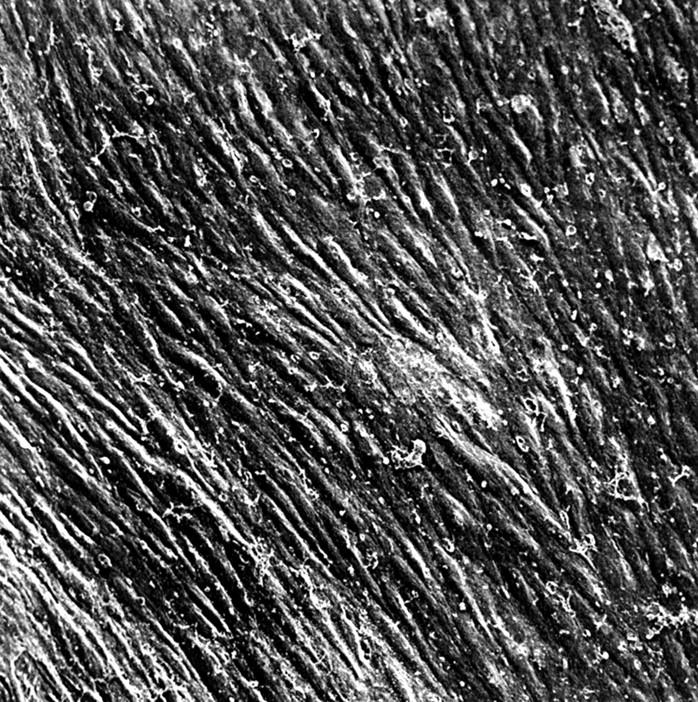
New Zealand white rabbit 12 months after implantation; scanning electron microscopic view of the aortic neointima continuously covered by confluent endothelial cells. Note that coagulation of plasma components and thrombocytes on the surface was caused by inadequate preparation of the specimen. Original magnification ×330.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H. D., Beekman R. H., 3rd, Garson A., Jr, Hijazi Z. M., Mullins C., O'Laughlin M. P., Taubert K. A. Pediatric therapeutic cardiac catheterization: a statement for healthcare professionals from the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 1998 Feb 17;97(6):609–625. doi: 10.1161/01.cir.97.6.609. [DOI] [PubMed] [Google Scholar]
- Andrews N. C. Disorders of iron metabolism. N Engl J Med. 1999 Dec 23;341(26):1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- Auerbach M., Winchester J., Wahab A., Richards K., McGinley M., Hall F., Anderson J., Briefel G. A randomized trial of three iron dextran infusion methods for anemia in EPO-treated dialysis patients. Am J Kidney Dis. 1998 Jan;31(1):81–86. doi: 10.1053/ajkd.1998.v31.pm9428456. [DOI] [PubMed] [Google Scholar]
- Auerbach M., Witt D., Toler W., Fierstein M., Lerner R. G., Ballard H. Clinical use of the total dose intravenous infusion of iron dextran. J Lab Clin Med. 1988 May;111(5):566–570. [PubMed] [Google Scholar]
- Benson L. N., Hamilton F., Dasmahapatra H., Rabinowitch M., Coles J. C., Freedom R. M. Percutaneous implantation of a balloon-expandable endoprosthesis for pulmonary artery stenosis: an experimental study. J Am Coll Cardiol. 1991 Nov 1;18(5):1303–1308. doi: 10.1016/0735-1097(91)90552-k. [DOI] [PubMed] [Google Scholar]
- Benson L. N., Nykanen D., Freedom R. M. Endovascular stents in pediatric cardiovascular medicine. J Interv Cardiol. 1995 Dec;8(6 Suppl):767–775. doi: 10.1111/j.1540-8183.1995.tb00929.x. [DOI] [PubMed] [Google Scholar]
- Chatelain P., Meier B., Friedli B. Stenting of superior vena cava and inferior vena cava for symptomatic narrowing after repeated atrial surgery for D-transposition of the great vessels. Br Heart J. 1991 Dec;66(6):466–468. doi: 10.1136/hrt.66.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo A., Karvouni E. Biodegradable stents : "fulfilling the mission and stepping away". Circulation. 2000 Jul 25;102(4):371–373. doi: 10.1161/01.cir.102.4.371. [DOI] [PubMed] [Google Scholar]
- Ebeid M. R., Prieto L. R., Latson L. A. Use of balloon-expandable stents for coarctation of the aorta: initial results and intermediate-term follow-up. J Am Coll Cardiol. 1997 Dec;30(7):1847–1852. doi: 10.1016/s0735-1097(97)00408-7. [DOI] [PubMed] [Google Scholar]
- Fogelman R., Nykanen D., Smallhorn J. F., McCrindle B. W., Freedom R. M., Benson L. N. Endovascular stents in the pulmonary circulation. Clinical impact on management and medium-term follow-up. Circulation. 1995 Aug 15;92(4):881–885. doi: 10.1161/01.cir.92.4.881. [DOI] [PubMed] [Google Scholar]
- Hoffmann R., Mintz G. S., Popma J. J., Satler L. F., Pichard A. D., Kent K. M., Walsh C., Mackell P., Leon M. B. Chronic arterial responses to stent implantation: a serial intravascular ultrasound analysis of Palmaz-Schatz stents in native coronary arteries. J Am Coll Cardiol. 1996 Nov 1;28(5):1134–1139. doi: 10.1016/S0735-1097(96)00278-1. [DOI] [PubMed] [Google Scholar]
- Ing F. F., Grifka R. G., Nihill M. R., Mullins C. E. Repeat dilation of intravascular stents in congenital heart defects. Circulation. 1995 Aug 15;92(4):893–897. doi: 10.1161/01.cir.92.4.893. [DOI] [PubMed] [Google Scholar]
- Lincoff A. M., Furst J. G., Ellis S. G., Tuch R. J., Topol E. J. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol. 1997 Mar 15;29(4):808–816. doi: 10.1016/s0735-1097(96)00584-0. [DOI] [PubMed] [Google Scholar]
- Magee A. G., Brzezinska-Rajszys G., Qureshi S. A., Rosenthal E., Zubrzycka M., Ksiazyk J., Tynan M. Stent implantation for aortic coarctation and recoarctation. Heart. 1999 Nov;82(5):600–606. doi: 10.1136/hrt.82.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr P., Fischer A., Brauer H., Denk A., Haude M., Erbel R. Biophysikalische Prüfung koronarer Stents: Welche Faktoren beeinflussen das Dilatations- und Recoilverhalten? Z Kardiol. 2000 Jun;89(6):513–521. doi: 10.1007/s003920070223. [DOI] [PubMed] [Google Scholar]
- Mendelsohn A. M., Bove E. L., Lupinetti F. M., Crowley D. C., Lloyd T. R., Fedderly R. T., Beekman R. H., 3rd Intraoperative and percutaneous stenting of congenital pulmonary artery and vein stenosis. Circulation. 1993 Nov;88(5 Pt 2):II210–II217. [PubMed] [Google Scholar]
- Morrow W. R., Palmaz J. C., Tio F. O., Ehler W. J., VanDellen A. F., Mullins C. E. Re-expansion of balloon-expandable stents after growth. J Am Coll Cardiol. 1993 Dec;22(7):2007–2013. doi: 10.1016/0735-1097(93)90791-x. [DOI] [PubMed] [Google Scholar]
- O'Laughlin M. P., Perry S. B., Lock J. E., Mullins C. E. Use of endovascular stents in congenital heart disease. Circulation. 1991 Jun;83(6):1923–1939. doi: 10.1161/01.cir.83.6.1923. [DOI] [PubMed] [Google Scholar]
- O'Laughlin M. P., Slack M. C., Grifka R. G., Perry S. B., Lock J. E., Mullins C. E. Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation. 1993 Aug;88(2):605–614. doi: 10.1161/01.cir.88.2.605. [DOI] [PubMed] [Google Scholar]
- Redington A. N., Hayes A. M., Ho S. Y. Transcatheter stent implantation to treat aortic coarctation in infancy. Br Heart J. 1993 Jan;69(1):80–82. doi: 10.1136/hrt.69.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redington A. N., Weil J., Somerville J. Self expanding stents in congenital heart disease. Br Heart J. 1994 Oct;72(4):378–383. doi: 10.1136/hrt.72.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E., Qureshi S. A., Tynan M. Stent implantation for aortic recoarctation. Am Heart J. 1995 Jun;129(6):1220–1221. doi: 10.1016/0002-8703(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Shaffer K. M., Mullins C. E., Grifka R. G., O'Laughlin M. P., McMahon W., Ing F. F., Nihill M. R. Intravascular stents in congenital heart disease: short- and long-term results from a large single-center experience. J Am Coll Cardiol. 1998 Mar 1;31(3):661–667. doi: 10.1016/s0735-1097(97)00535-4. [DOI] [PubMed] [Google Scholar]
- Suárez de Lezo J., Pan M., Romero M., Medina A., Segura J., Lafuente M., Pavlovic D., Hernández E., Melián F., Espada J. Immediate and follow-up findings after stent treatment for severe coarctation of aorta. Am J Cardiol. 1999 Feb 1;83(3):400–406. doi: 10.1016/s0002-9149(98)00877-7. [DOI] [PubMed] [Google Scholar]
- Suárez de Lezo J., Pan M., Romero M., Medina A., Segura J., Pavlovic D., Martinez C., Tejero I., Perez Navero J., Torres F. Balloon-expandable stent repair of severe coarctation of aorta. Am Heart J. 1995 May;129(5):1002–1008. doi: 10.1016/0002-8703(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Tamai H., Igaki K., Kyo E., Kosuga K., Kawashima A., Matsui S., Komori H., Tsuji T., Motohara S., Uehata H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000 Jul 25;102(4):399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- Thanopoulos B. D., Hadjinikolaou L., Konstadopoulou G. N., Tsaousis G. S., Triposkiadis F., Spirou P. Stent treatment for coarctation of the aorta: intermediate term follow up and technical considerations. Heart. 2000 Jul;84(1):65–70. doi: 10.1136/heart.84.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. J., Mullins C. E., Nihill M. R., Grifka R. G., Vick G. W., 3rd Use of intravascular stents in systemic venous and systemic venous baffle obstructions. Short-term follow-up results. Circulation. 1995 Jun 15;91(12):2948–2954. doi: 10.1161/01.cir.91.12.2948. [DOI] [PubMed] [Google Scholar]
- Yamawaki T., Shimokawa H., Kozai T., Miyata K., Higo T., Tanaka E., Egashira K., Shiraishi T., Tamai H., Igaki K. Intramural delivery of a specific tyrosine kinase inhibitor with biodegradable stent suppresses the restenotic changes of the coronary artery in pigs in vivo. J Am Coll Cardiol. 1998 Sep;32(3):780–786. doi: 10.1016/s0735-1097(98)00312-x. [DOI] [PubMed] [Google Scholar]
- van Beusekom H. M., Whelan D. M., Hofma S. H., Krabbendam S. C., van Hinsbergh V. W., Verdouw P. D., van der Giessen W. J. Long-term endothelial dysfunction is more pronounced after stenting than after balloon angioplasty in porcine coronary arteries. J Am Coll Cardiol. 1998 Oct;32(4):1109–1117. doi: 10.1016/s0735-1097(98)00348-9. [DOI] [PubMed] [Google Scholar]
- van der Giessen W. J., Lincoff A. M., Schwartz R. S., van Beusekom H. M., Serruys P. W., Holmes D. R., Jr, Ellis S. G., Topol E. J. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996 Oct 1;94(7):1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]