Abstract
OBJECTIVE—To investigate the value of coronary pressure derived fractional flow reserve (FFR) measurements in supporting decisions about medical or surgical treatment in patients with angiographically equivocal left main coronary artery stenosis. DESIGN—A two centre prospective single cohort follow up study. INTERVENTIONS—FFR of the left main coronary artery was determined in 54 consecutive patients with angiographically equivocal left main coronary artery disease. If FFR was ⩾ 0.75, medical treatment was chosen; if FFR was < 0.75, surgical treatment was chosen. MAIN OUTCOME MEASURES—Freedom from death, myocardial infarction, or any coronary revascularisation procedure. RESULTS—In 24 patients (44%), FFR was ⩾ 0.75 and medical treatment was chosen (medical group). In the remaining 30 patients (56%), FFR was < 0.75 and bypass surgery was performed (surgical group). Mean (SD) follow up was 29 (15) months (range 12-65 months). Survival among patients at three years of follow up was 100% in the medical group and 97% in the surgical group. Event-free survival was 76% in the medical group and 83% in the surgical group. CONCLUSIONS—FFR supports decision making in equivocal left main coronary artery disease. If FFR is below 0.75, the decision for bypass surgery is supported. If FFR is above 0.75, a conservative approach is justified. Keywords: coronary artery disease; left main coronary artery; fractional flow reserve; coronary artery bypass
Full Text
The Full Text of this article is available as a PDF (181.1 KB).
Figure 1 .
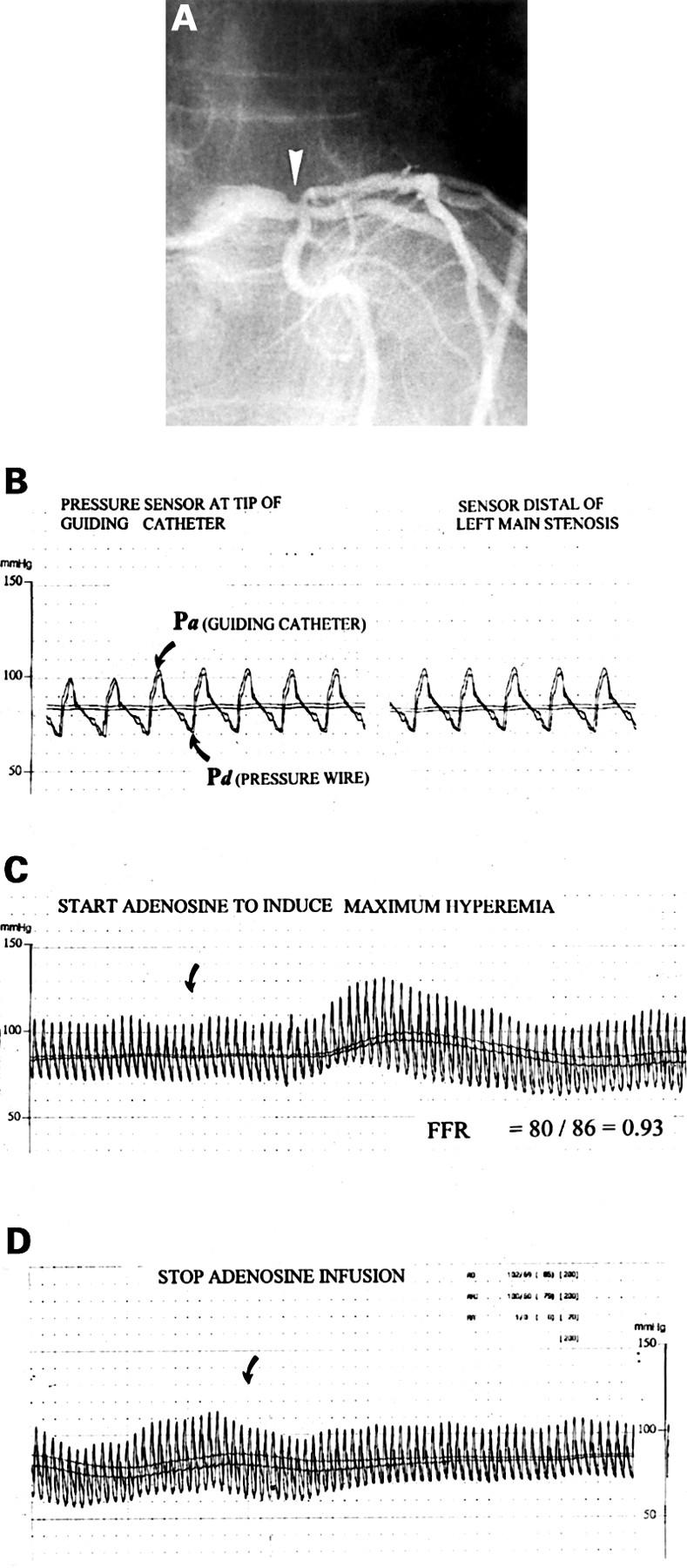
(A) Coronary angiogram in the right anterior oblique projection of a 58 year old man with anginal complaints, a reversible defect on exercise perfusion scintigraphy in the anterior wall, a 40% stenosis (arrow) at the transition of the distal left main coronary artery (LM), and an 80% stenosis of the proximal left anterior descending coronary artery (LAD). The dilemma was to decide whether the ischaemia was caused by the proximal LAD disease only or also by the left main stenosis, and consequently if bypass surgery should be performed or only percutaneous transluminal coronary angioplasty (PTCA) of the proximal LAD. (B) Paper speed is 25 mm/s. The pressure wire is advanced until the sensor is close to the tip of the guiding catheter to confirm that two identical signals are obtained. Pa is the pressure as measured by the guiding catheter and Pd the pressure as measured by the pressure wire. The pressure wire is then advanced across the LM stenosis into the large intermediate branch and no gradient is observed at rest. (C) Paper speed is 5 mm/s. About 20 seconds after the start of the intravenous adenosine infusion (140 µg/kg/min) a pressure gradient gradually develops (arrow) and at steady state, maximum coronary hyperaemic myocardial fractional flow reserve is calculated by the ratio between Pd and Pa [80/86 = 0.93], indicating that this left main lesion in itself was not significant from a functional point of view. (D) About 30 seconds after cessation of adenosine infusion the pressure gradient disappears and returns to baseline. In this patient bypass surgery was deferred and he underwent PTCA of the proximal left anterior descending stenosis only. FFR of the LAD was 0.63 before and 0.94 after PTCA.
Figure 2 .
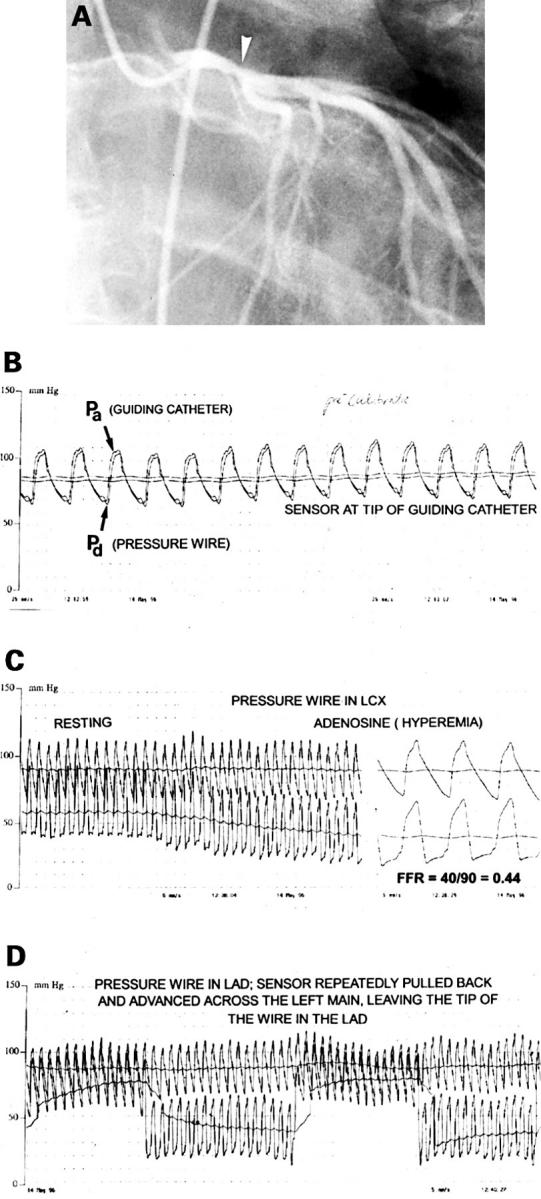
(A) Coronary angiogram in the right anterior oblique projection from a 36 year old man who experienced an anterior wall myocardial infarct, successfully treated by thrombolysis. At angiography only a 40% stenosis (arrow) of the distal left main coronary artery (LM) was visible, and there was mild hypokinesia of the anterior wall segments. Perfusion scintigraphy performed several weeks later was negative. The dilemma was whether to perform bypass surgery or to leave this lesion untreated. (B) A pressure wire is advanced to the tip of the coronary catheter, and equal pressures are registered at that position by the guiding catheter (Pa) and the pressure sensor (Pd ). (C) The pressure wire is advanced across the left main stenosis into the left circumflex coronary artery and a large gradient is observed at rest. After start of intravenous adenosine infusion (140 µg/kg/min), this gradient gradually further increases and at steady state maximum coronary hyperaemic myocardial fractional flow reserve is calculated by the ratio between Pd and Pa [40/90 = 0.44], indicating that this left main lesion is physiologically highly significant. (D) Shows how the wire is positioned in the left anterior descending coronary artery and slowly withdrawn and advanced across the left main stenosis. Because the pressure sensor is located at 3 cm from the tip of the wire, this pull-back/push-up procedure can be performed repeatedly under fluoroscopy. It confirms the presence and exact location of a stenosis. This patient was treated by bypass surgery.
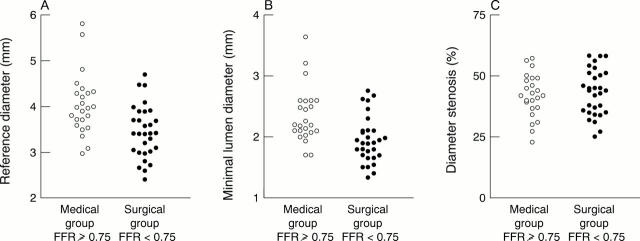
Figure 3 .
Relation between reference diameter, minimum lumen diameter, and per cent diameter stenosis versus myocardial fractional flow reserve. Empty circles represent the 24 patients in the medical group with left main coronary artery FFR ⩾ 0.75; filled circles represent the 30 patients in the surgical group with left main coronary artery FFR < 0.75.
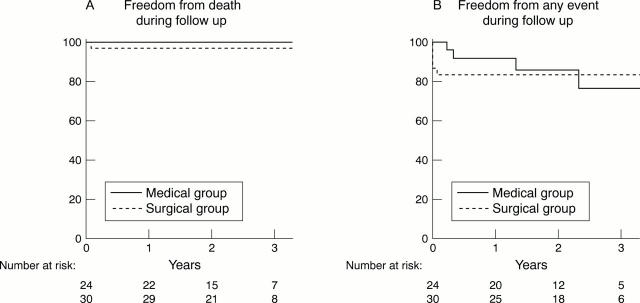
Figure 4 .
Estimated survival and event-free survival curves after three years of follow up (Kaplan-Meier) for the two study groups. The medical group consisted of 24 patients with an equivocal left main coronary artery stenosis in whom bypass surgery was deferred on the basis of a left main coronary artery FFR of ⩾ 0.75. The surgical group consisted of 30 patients with an equivocal left main coronary artery stenosis in whom bypass surgery was performed on the basis of a left main coronary artery FFR of < 0.75. Numbers below the x axis represent patients at risk at one, two, and three years of follow up.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bech G. J., De Bruyne B., Bonnier H. J., Bartunek J., Wijns W., Peels K., Heyndrickx G. R., Koolen J. J., Pijls N. H. Long-term follow-up after deferral of percutaneous transluminal coronary angioplasty of intermediate stenosis on the basis of coronary pressure measurement. J Am Coll Cardiol. 1998 Mar 15;31(4):841–847. doi: 10.1016/s0735-1097(98)00050-3. [DOI] [PubMed] [Google Scholar]
- Beller G. A., Zaret B. L. Contributions of nuclear cardiology to diagnosis and prognosis of patients with coronary artery disease. Circulation. 2000 Mar 28;101(12):1465–1478. doi: 10.1161/01.cir.101.12.1465. [DOI] [PubMed] [Google Scholar]
- Cameron A., Kemp H. G., Jr, Fisher L. D., Gosselin A., Judkins M. P., Kennedy J. W., Lesperance J., Mudd J. G., Ryan T. J., Silverman J. F. Left main coronary artery stenosis: angiographic determination. Circulation. 1983 Sep;68(3):484–489. doi: 10.1161/01.cir.68.3.484. [DOI] [PubMed] [Google Scholar]
- Caracciolo E. A., Davis K. B., Sopko G., Kaiser G. C., Corley S. D., Schaff H., Taylor H. A., Chaitman B. R. Comparison of surgical and medical group survival in patients with left main coronary artery disease. Long-term CASS experience. Circulation. 1995 May 1;91(9):2325–2334. doi: 10.1161/01.cir.91.9.2325. [DOI] [PubMed] [Google Scholar]
- Chaitman B. R., Fisher L. D., Bourassa M. G., Davis K., Rogers W. J., Maynard C., Tyras D. H., Berger R. L., Judkins M. P., Ringqvist I. Effect of coronary bypass surgery on survival patterns in subsets of patients with left main coronary artery disease. Report of the Collaborative Study in Coronary Artery Surgery (CASS). Am J Cardiol. 1981 Oct;48(4):765–777. doi: 10.1016/0002-9149(81)90156-9. [DOI] [PubMed] [Google Scholar]
- Chaitman B. R., Rogers W. J., Davis K., Tyras D. H., Berger R., Bourassa M. G., Fisher L., StoverHertzberg V., Judkins M. P., Mock M. B. Operative risk factors in patients with left main coronary-artery disease. N Engl J Med. 1980 Oct 23;303(17):953–957. doi: 10.1056/NEJM198010233031701. [DOI] [PubMed] [Google Scholar]
- Erbel R., Ge J., Bockisch A., Kearney P., Görge G., Haude M., Schümann D., Zamorano J., Rupprecht H. J., Meyer J. Value of intracoronary ultrasound and Doppler in the differentiation of angiographically normal coronary arteries: a prospective study in patients with angina pectoris. Eur Heart J. 1996 Jun;17(6):880–889. doi: 10.1093/oxfordjournals.eurheartj.a014969. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fyke F. E., 3rd, Brown M. L., Lapeyre A. C., 3rd, Zinsmeister A. R., Clements I. P. Comparison of exercise performance in left main and three-vessel coronary artery disease. Cathet Cardiovasc Diagn. 1991 Jan;22(1):14–20. doi: 10.1002/ccd.1810220104. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Isshiki T., Ikari Y., Hara K., Saeki F., Tamura T., Yamaguchi T., Suma H. Effects of competitive blood flow on arterial graft patency and diameter. Medium-term postoperative follow-up. J Thorac Cardiovasc Surg. 1996 Feb;111(2):399–407. doi: 10.1016/s0022-5223(96)70449-x. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Kishel J., Kent K. M., Ronan J. A., Jr, Ross A. M., Roberts W. C. Accuracy of angiographic determination of left main coronary arterial narrowing. Angiographic--histologic correlative analysis in 28 patients. Circulation. 1981 May;63(5):1056–1064. doi: 10.1161/01.cir.63.5.1056. [DOI] [PubMed] [Google Scholar]
- Johnston P. W., Fort S., Cohen E. A. Noncritical disease of the left main coronary artery: limitations of angiography and the role of intravascular ultrasound. Can J Cardiol. 1999 Mar;15(3):297–302. [PubMed] [Google Scholar]
- Jánosi A., Vértes A. Exercise testing and left main coronary artery stenosis. Can patients with left main disease be identified? Chest. 1991 Jul;100(1):227–229. doi: 10.1378/chest.100.1.227. [DOI] [PubMed] [Google Scholar]
- Kern M. J., Deligonul U., Tatineni S., Serota H., Aguirre F., Hilton T. C. Intravenous adenosine: continuous infusion and low dose bolus administration for determination of coronary vasodilator reserve in patients with and without coronary artery disease. J Am Coll Cardiol. 1991 Sep;18(3):718–729. doi: 10.1016/0735-1097(91)90795-b. [DOI] [PubMed] [Google Scholar]
- Lust R. M., Zeri R. S., Spence P. A., Hopson S. B., Sun Y. S., Otaki M., Jolly S. R., Mehta P. M., Chitwood W. R., Jr Effect of chronic native flow competition on internal thoracic artery grafts. Ann Thorac Surg. 1994 Jan;57(1):45–50. doi: 10.1016/0003-4975(94)90363-8. [DOI] [PubMed] [Google Scholar]
- Nishimura R. A., Higano S. T., Holmes D. R., Jr Use of intracoronary ultrasound imaging for assessing left main coronary artery disease. Mayo Clin Proc. 1993 Feb;68(2):134–140. doi: 10.1016/s0025-6196(12)60160-8. [DOI] [PubMed] [Google Scholar]
- Pijls N. H., De Bruyne B., Peels K., Van Der Voort P. H., Bonnier H. J., Bartunek J Koolen J. J., Koolen J. J. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996 Jun 27;334(26):1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- Pijls N. H., Van Gelder B., Van der Voort P., Peels K., Bracke F. A., Bonnier H. J., el Gamal M. I. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995 Dec 1;92(11):3183–3193. doi: 10.1161/01.cir.92.11.3183. [DOI] [PubMed] [Google Scholar]
- Takaro T., Peduzzi P., Detre K. M., Hultgren H. N., Murphy M. L., van der Bel-Kahn J., Thomsen J., Meadows W. R. Survival in subgroups of patients with left main coronary artery disease. Veterans Administration Cooperative Study of Surgery for Coronary Arterial Occlusive Disease. Circulation. 1982 Jul;66(1):14–22. doi: 10.1161/01.cir.66.1.14. [DOI] [PubMed] [Google Scholar]
- Wolfhard U., Görge G., Konorza T., Haude M., Ge J., Piotrowski J. A., Splittgerber F. H., Sadony V., Erbel R. Intravascular ultrasound (IVUS) examination reverses therapeutic decision from percutaneous intervention to a surgical approach in patients with alterations of the left main stem. Thorac Cardiovasc Surg. 1998 Oct;46(5):281–284. doi: 10.1055/s-2007-1010239. [DOI] [PubMed] [Google Scholar]
- van der Voort P. H., van Hagen E., Hendrix G., van Gelder B., Bech J. W., Pijls N. H. Comparison of intravenous adenosine to intracoronary papaverine for calculation of pressure-derived fractional flow reserve. Cathet Cardiovasc Diagn. 1996 Oct;39(2):120–125. doi: 10.1002/(SICI)1097-0304(199610)39:2<120::AID-CCD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]