Abstract
LXRα is a nuclear receptor that has previously been shown to regulate the metabolic conversion of cholesterol to bile acids. Here we define a role for this transcription factor in the control of cellular cholesterol efflux. We demonstrate that retroviral expression of LXRα in NIH 3T3 fibroblasts or RAW264.7 macrophages and/or treatment of these cells with oxysterol ligands of LXR results in 7- to 30-fold induction of the mRNA encoding the putative cholesterol/phospholipid transporter ATP-binding cassette (ABC)A1. In contrast, induction of ABCA1 mRNA in response to oxysterols is attenuated in cells that constitutively express dominant-negative forms of LXRα or LXRβ that lack the AF2 transcriptional activation domain. We further demonstrate that expression of LXRα in NIH 3T3 fibroblasts and/or treatment of these cells with oxysterols is sufficient to stimulate cholesterol efflux to extracellular apolipoprotein AI. The ability of oxysterol ligands of LXR to stimulate efflux is dramatically reduced in Tangier fibroblasts, which carry a loss of function mutation in the ABCA1 gene. Taken together, these results indicate that cellular cholesterol efflux is controlled, at least in part, at the level of transcription by a nuclear receptor–signaling pathway. They suggest a model in which activation of LXRs by oxysterols in response to cellular sterol loading leads to induction of the ABCA1 transporter and the stimulation of lipid efflux to extracellular acceptors. These findings have important implications for our understanding of mammalian cholesterol homeostasis and suggest new opportunities for pharmacological regulation of cellular lipid metabolism.
Human and murine macrophages express cell surface receptors that bind and then rapidly internalize oxidized or modified (e.g., acetylated) low density lipoprotein (LDL) (1). This rapid and unregulated uptake of the cholesterol-rich LDL results in the formation of intracellular cholesteryl ester inclusions that give the macrophages a foamy appearance (2). Such cholesteryl ester-rich foam cells have been identified in both early and advanced atherosclerotic lesions of humans and animals and are thought to play a critical role in the development of the disease (3, 4). Thus, the differential expression of specific genes in lipid loaded macrophages is likely to play an important role in the pathology of atherosclerosis.
Recent studies have identified two genes, ATP-binding cassette (ABC)A1 (5) and ABCG1 (6, 7), whose mRNAs are induced 7- to >100-fold in lipid-loaded macrophages. In earlier publications, ABCA1 was referred to as ABC1, whereas ABCG1 was referred to as either ABC8 (murine) or white (human). ABCA1 and ABCG1 are members of the ATP binding cassette (ABC) superfamily of transporter proteins that bind and hydrolyze ATP to provide energy for transmembrane transport (8–10). The ABCA1 gene encodes a full transporter protein containing 12 putative transmembrane domains and two ABCs (11), whereas the ABCG1 gene encodes a half transporter protein containing six putative transmembrane domains and one ABC (12, 13).
Studies from a number of laboratories have provided compelling evidence that the ABCA1 protein facilitates the efflux of cellular cholesterol to extracellular apolipoprotein AI (apoAI) or high density lipoprotein (HDL). Defective efflux of cellular lipids to serum apolipoproteins is thought to account for the abnormally low plasma HDL levels noted in ABCA1 null mice (14) and in patients with Tangier disease or familial HDL deficiency (15), in which the ABCA1 gene is mutated (16–19). Defective lipid efflux is also likely to underlie the accumulation of lipid-loaded reticulo-endothelial cells and cholesteryl ester-loaded macrophages in the tonsils of patients with Tangier disease (15), as well as the presence of excess foamy type II pneumocytes and lipid-loaded macrophages in the lungs of ABCA1 null mice (14). In support of this hypothesis, it has been shown that treatment of cells with antisense oligonucleotides to ABCA1 (16, 17) leads to a decrease in the efflux of cellular cholesterol and phospholipids to HDL or apoAI in the medium. Moreover, fibroblasts derived either from patients with Tangier disease (16–19) or from ABCA1 null mice (20) exhibit a >60% reduction in the rate of efflux of cholesterol and/or phospholipids to HDL or apoAI. Studies have also shown that absorption of dietary cholesterol is increased in ABCA1 null mice, consistent with an important role for this transporter protein in cholesterol absorption (14). Taken together, these observations imply a central role for ABCA1 in regulation of cholesterol and/or phospholipid efflux to extracellular acceptors. Although the physiological function of ABCG1 is less well understood, antisense oligonucleotides to ABCG1 have also been reported to inhibit lipid efflux (6).
The ability of ABCA1 to facilitate cholesterol efflux suggests that it may be a useful target for intervention in human disease. However, the molecular mechanism that links cellular lipid loading to the induction of ABCA1 expression is not known. Here we demonstrate that the nuclear receptor LXRα and its oxysterol ligands regulate expression of ABCA1 in macrophages and fibroblasts. Moreover, we show that ligand activation of LXRα is sufficient to promote cholesterol efflux to extracellular acceptors through ABCA1. These observations suggest that cellular cholesterol efflux is controlled, at least in part, by a nuclear receptor signaling pathway.
Materials and Methods
Reagents.
Acetylated LDL (Ac-LDL) and highly oxidized LDL (Ox-LDL) were prepared from human LDL as described (7). Cholesterol (Steraloids, Wilton, NH) and oxysterols (Sigma) were dissolved in ethanol before addition to cells (<1 μl/ml). ApoAI (Intracel) was resuspended in PBS, quick frozen and stored in aliquots at −80°C. LG268 was a gift from R. Heyman (Ligand Pharmaceuticals). [3H]Cholesterol (45 Ci/mmol) was from NEN.
Cell Culture and RNA Analysis.
Human monocytes were isolated from peripheral blood, allowed to adhere to plastic dishes, and cultured in autologous serum for 8 days as described (7). RAW264.7 macrophages and NIH 3T3 fibroblasts (ATCC) were grown in DMEM supplemented with 10% FBS or 10% calf serum, respectively. Transformed normal and Tangier primary skin fibroblasts (21), a gift of John F. Oram (University of Washington), were cultured in DMEM plus 10% FBS. Cells were washed with PBS and cultured for 16–24 h in medium A (DMEM containing 10% lipoprotein-deficient FBS). Cells were treated with a lipid mixture [Ac-LDL (100 μg/ml), 25-hydroxycholesterol (OHC) (1 μg/ml), and cholesterol (10 μg/ml)], specific lipoproteins or lipids as indicated. Cycloheximide (10 μg/ml) or actinomycin D (5 μg/ml) were used as described (7). For Northern blots, 10 μg of total RNA, isolated from cells using Trizol Reagent (Life Technologies), was separated by gel electrophoresis, transferred to nylon membranes, and hybridized with [α-32P]dCTP-labeled DNA probes (22). The relative abundance of transcripts was determined using a PhosphorImager (Molecular Dynamics) and standardized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Stable Cell Lines.
cDNAs encoding full-length murine LXRα (23), an AF-2 deletion mutant of LXRα (amino acids 1–435; LXRαΔAF2), or an AF-2 deletion mutant of LXRβ (amino acids 1–437; LXRβΔAF2) were cloned into the pBabe-Puro expression vector (24) and packaged into retrovirus by transient transfection of Phoenix E cells as described (22, 25). NIH 3T3 cells or RAW 264.7 macrophages were infected at 50% confluence with equal titers of recombinant retrovirus. Stable cell lines were selected with puromycin (2 μg/ml).
Cholesterol Efflux.
Efflux assays were modified from those described (26). Briefly, cells were plated at 50% confluence in 24-well plates. On day 2, the cells were washed and incubated for 24 h in medium A supplemented with an ACAT inhibitor (58–035; 2 μg/ml) and [3H]cholesterol (1.0 μCi/ml). Oxysterols (1.0 μg/ml) and/or LG268 (50 nM) were added to the cells as indicated. On day 3, the cells were washed twice with PBS and then incubated for 2–4 h in fresh medium, devoid of radiolabeled cholesterol, but containing the indicated oxysterol or LG268. The cells were rewashed before addition of 1.0 ml medium B (DMEM containing 0.2% BSA) in the absence or presence of apoAI (15 μg/ml). After a 4-h incubation, the medium was removed and centrifuged at 14,000 × g for 10 min, and the radioactivity was determined by liquid scintillation counting. The cells were dissolved in isopropanol and an aliquot used to determine total cell-associated radioactivity. The apoAI-dependent efflux of radioactive cholesterol from the cells into the medium was determined as a percentage of the total radioactivity in the cells and medium for each condition. Each efflux assay was performed in triplicate. Efflux was linear for at least 8 h (data not shown).
Results
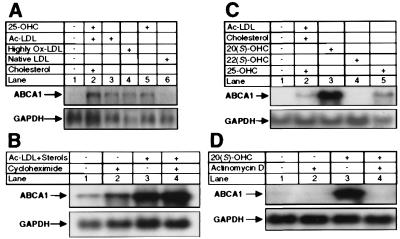
We have previously shown that incubation of human monocyte macrophages with a lipid mixture (Ac-LDL, 25-OHC, and cholesterol) results in the accumulation of large amounts of cholesteryl ester and a ≥100-fold increase in the mRNA encoding ABCG1 (7). We investigated whether other members of the ABC family might also be induced under these conditions. The data of Fig. 1A demonstrate that this same treatment results in a ≥20-fold induction of mRNA encoding ABCA1 (lane 2 vs. 1). The degree of induction of ABCA1 mRNA was clearly dependent on the type of exogenously added lipid. Ac-LDL, Ox-LDL, and 25-OHC were more potent inducers of ABCA1 mRNA levels than LDL (Fig. 1A). A significant induction of ABCA1 mRNA was observed within 4–8 h, and this induction was not blocked by treatment with cycloheximide at concentrations that inhibited protein synthesis by >98% (Fig. 1B and data not shown). ABCA1 mRNA levels were also induced when murine RAW264.7 cells, murine peritoneal macrophages, or human THP-1 cells were incubated with the lipid mixture or with 20(S)- or 25-OHC (Fig. 1C and data not shown). Importantly, the latter two oxysterols are known ligands of the nuclear receptor LXR (27–29). In contrast, ABCA1 mRNA levels were not increased after the addition of 22(S)-OHC (Fig. 1C), an oxysterol that is unable to activate LXR. The induction of ABCA1 mRNA in response to 20(S)-OHC was attenuated when the cells were coincubated with actinomycin D, an inhibitor of transcription (Fig. 1D).
Figure 1.
Lipoproteins and oxysterols induce ABCA1 mRNA levels in human and murine macrophages. Total RNA (10 μg per lane) was separated on a 1% agarose/formaldehyde gel, transferred to nylon, and hybridized to radiolabeled cDNA probes. Membranes were probed with GAPDH cDNA to ensure equivalent loading and integrity of RNA. (A) Human macrophages were incubated for 48 h in medium A supplemented with 100 μM mevalonic acid. A lipid mixture (100 μg/ml Ac-LDL, 1 μg/ml 25-OHC, and 10 μg/ml cholesterol), 100 μg/ml Ac-LDL, Ox-LDL or LDL, or 4 μg/ml 25-OHC was added to the cells as indicated. (B) Human macrophages were incubated for 8 h in medium A in the absence or presence of the lipid mixture or cycloheximide (10 μg/ml) as indicated. (C) RAW264.7 macrophages were incubated for 48 h in medium A supplemented with 100 μM mevalonic acid in the absence or presence of lipid mixture or oxysterol (10 μM). (D) RAW264.7 cells were incubated for 8 h in medium A supplemented with 20(S)-OHC and/or actinomycin D (5 μg/ml), as indicated.
The results presented in Fig. 1 strongly suggest that specific oxysterols activate transcription of the ABCA1 gene by a process that is independent of continued protein synthesis. Moreover, the stereochemical requirements for 22-OHC induction of ABCA1 is consistent with an LXR-mediated process. The induction of ABCA1 mRNA by various lipids is paralleled by similar changes in the expression of mRNA encoding ABCG1 (data not shown), which we have recently shown to be a target for LXR regulation (7). Coupled with the data of Fig. 1, these observations strongly suggest that the coordinate induction of ABCA1 and ABCG1 mRNA by oxysterols is mediated by the nuclear receptors LXRα and/or LXRβ.
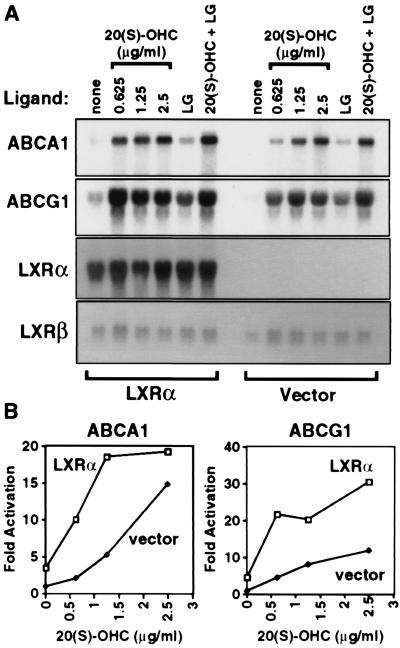
To directly address the ability of LXRα to regulate ABCA1 expression, RAW264.7 macrophages were transduced with a retroviral expression vector encoding LXRα (RAW-LXRα) or with vector alone (RAW-vector) as described in Materials and Methods. Northern blot analysis confirmed abundant expression of LXRα mRNA in the RAW-LXRα cells but not in the RAW-vector cells (Fig. 2A). Expression of LXRβ mRNA was similar in both cell lines. RAW-LXRα and RAW-vector cells were incubated for 24 h in the absence or presence of the LXR ligand 20(S)-OHC, the retinoid X receptor (RXR)-specific ligand LG268, or both. As shown in Fig. 2A, mRNAs encoding ABCA1 and ABCG1 were very low in RAW-vector cells in the absence of oxysterol. By contrast, RAW-LXRα cells showed significant expression of both transcripts even in the absence of exogenously added oxysterol. Addition of 20(S)-OHC resulted in induction of ABCA1 and ABCG1 mRNAs in both cell types. However, maximal induction was observed at an oxysterol concentration of ≤1.25 μg/ml in the RAW-LXRα cells as compared with a concentration of 2.5 μg/ml in RAW-vector cells (Fig. 2B). Thus, the dose-response was shifted significantly to the left in the cells that overexpressed LXRα. Addition of LG268 also resulted in a small but significant induction of both mRNAs (Fig. 2A). This latter result is consistent with the finding that LXR/RXR is a permissive heterodimer and activates transcription in response to ligand for either partner (23, 30). Taken together, these observations are consistent with an important role for both LXRs and oxysterols in transcriptional regulation of macrophage ABCA1 and ABCG1 expression. In control cells, oxysterols presumably induce expression of these mRNAs through LXRβ or and/or low levels of endogenous LXRα (see below). The oxysterol response is significantly enhanced through ectopic overexpression of LXRα.
Figure 2.
Retroviral expression of LXRα in RAW264.7 cells facilitates the induction of ABCA1 and ABCG1 mRNA in response to LXR and RXR ligands. (A) RAW264.7 cells were transduced with retroviral vector alone or LXRα expression vector as described in Materials and Methods. Cells were incubated for 24 h in medium A containing the indicated concentration of 20(S)-OHC and/or LG268 (50 nM). Northern analysis was performed as in Fig. 1. A cDNA probe for GAPDH was used as a control to ensure equivalent loading and integrity of RNA (not shown). (B) Quantitation of ABCA1 and ABCG1 mRNA levels shown in part A normalized for GAPDH expression. mRNA levels in untreated RAW-vector cells were arbitrarily assigned value of 1.0.
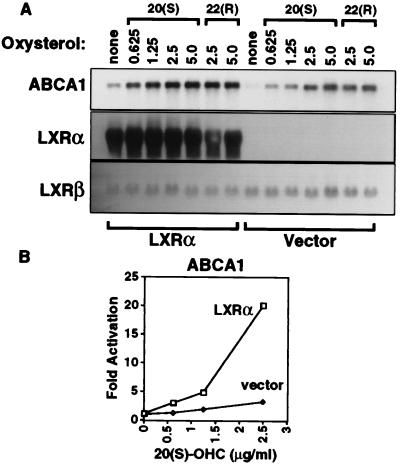
Next, we asked whether the LXR-dependent induction of ABCA1 was restricted to macrophages or whether it might be functional in other cell types. We examined the ability of retroviral LXRα expression to influence ABCA1 expression in NIH 3T3 fibroblasts. Northern blot analysis revealed high-level expression of LXRα mRNA in cells transduced with the LXRα expression vector (NIH-LXRα) but not in those transduced with the retroviral vector alone (NIH-vector; Fig. 3A). Expression of LXRβ mRNA was similar in both cell lines. ABCA1 mRNA levels were very low in control cells but increased significantly upon incubation of the cells with either 20(S)- or 22(R)-OHC. By contrast, stable expression of LXRα in NIH 3T3 cells resulted in significant expression of ABCA1 mRNA, even in the absence of exogenously added oxysterol (Fig. 3A). Addition of oxysterol ligands to these cells further induced ABCA1 expression. Comparison of the results obtained with NIH-vector and NIH-LXRα cells indicates that overexpression of LXRα shifts the dose-response to oxysterols to the left (Fig. 3B). Thus, in both RAW264.7 macrophages and NIH 3T3 cell fibroblasts, induction of ABCA1 mRNA depends on specific oxysterols and the level of expression of LXRα.
Figure 3.
Retroviral expression of LXRα in NIH 3T3 cells promotes the induction of ABCA1 mRNA in response to LXR and RXR ligands. (A) NIH 3T3 cells were transduced with retroviral vector alone or LXRα expression vector as described in Materials and Methods. Cells were incubated for 24 h in medium A containing the indicated concentration of 20(S)-OHC, 22(R)-OHC, and/or LG268 (50 nM). Northern analysis was performed as in Fig. 1. A cDNA probe for GAPDH was used as a control to ensure equivalent loading and integrity of RNA (not shown). (B) Quantitation of ABCA1 mRNA levels shown in A normalized for GAPDH expression. mRNA levels in untreated NIH-vector cells were arbitrarily assigned value of 1.0.
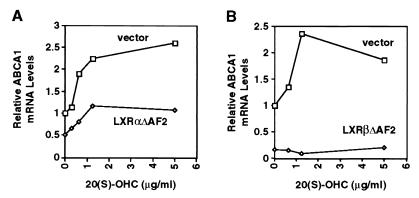
We hypothesized that the basal ability of oxysterols to induce ABCA1 expression in control RAW-vector and NIH-vector cells (as shown in Figs. 2 and 3) was attributable to endogenous LXRα and/or LXRβ. To verify this hypothesis, we used retroviral vectors to overexpress dominant negative forms of either LXRα or LXRβ (LXRαΔAF2, LXRβΔAF2; see Materials and Methods) in NIH 3T3 cells. These mutant receptors form heterodimers with RXR that bind to LXR response elements but fail to activate transcription in response to oxysterol ligands (data not shown). NIH-vector, NIH-LXRαΔAF2, and NIH-LXRβΔAF2 cells were treated for 24 h with increasing concentrations of 20(S)-OHC. As shown in Fig. 4, expression of either dominant-negative receptor significantly blunted the induction of ABCA1 by 20(S)-OHC. Expression of ABCA1 mRNA was lower in NIH-LXRαΔAF2 (Fig. 4A) and NIH-LXRβΔAF2 (Fig. 4B) cells compared with control cells at each dose of oxysterol tested. These data strongly suggest that oxysterol induction of ABCA1 expression in RAW and NIH 3T3 cells is mediated by endogenous LXRα and/or LXRβ.
Figure 4.
Expression of dominant negative LXRα or LXRβ in NIH 3T3 cells blunts induction of ABCA1 mRNA in response to 20(S)-OHC. NIH-3T3 cells were transduced with retroviral vector alone or LXRαΔAF2 or LXRβΔAF2 expression vectors as described in Materials and Methods. Cells were incubated for 24 h in medium A containing the indicated concentration of 20(S)-OHC. Northern analysis was performed as in Fig. 1. ABCA1 mRNA levels were quantitated by PhosphorImager and normalized to GAPDH. For each experiment the level of ABCA1 mRNA in NIH-vector cells in the absence of oxysterol was assigned a value of 1.0. (A) Effect of a dominant-negative LXRα on ABCA1 expression. (B) Effect of a dominant-negative LXRβ on ABCA1 expression.
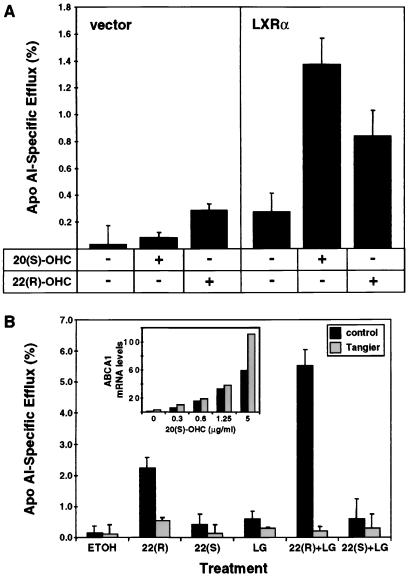
ABCA1 has recently been reported to play a role in regulating the efflux of cellular cholesterol and/or phospholipids to specific lipid acceptors (HDL or apoAI) in the medium (16, 17, 20). Our demonstration that expression of ABCA1 is controlled by LXR led us to hypothesize that the LXR pathway may be a central mechanism by which cellular lipid efflux is regulated. This idea was tested using the NIH-LXRα and NIH-vector cells described above. As shown in Fig. 5A, cholesterol efflux from murine NIH 3T3 cells to apoAI was significantly enhanced when the cells overexpressed LXRα from a retroviral vector (compare lane 4 vs. 1). Preincubation of the cells with 20(S)- or 22(R)-OHC for 28 h resulted in an increase in the rate of cholesterol efflux to apoAI (Fig. 5A, lanes 2 and 3 vs. 1, lanes 5 and 6 vs. 4). Under each condition, cholesterol efflux was greater from the LXRα-overexpressing cells than from the control cells (Fig. 5A). In data not shown, we have observed that LG268 also promotes efflux in these cells, whereas 22(S)-OHC (which is unable to activate LXRα) does not. Thus, ectopic expression of LXRα in fibroblasts is sufficient to alter the rate of cholesterol efflux to extracellular acceptors. The stereospecificity of the effect of 22-OHC on efflux strongly suggests that the activity of oxysterols in these assays is the result of ligand activation of LXR.
Figure 5.
Expression and activation of LXRα stimulates cholesterol efflux to apoAI through the ABCA1 pathway. (A) Oxysterol ligands of LXR stimulate cholesterol efflux from NIH 3T3 cells. NIH-vector and NIH-LXRα cells were plated in 24-well plates and incubated for 24 h in medium A supplemented with [3H]cholesterol in the absence or presence of the indicated oxysterol (1 μg/ml) or LG268 (LG, 50 nM). ApoAI-dependent cholesterol efflux to the medium was determined as described in Materials and Methods. Data are presented as a percentage (±SE) of the total radioactivity in cells and medium; each point is the numerical average of triplicate experiments. (B) Stimulation of cholesterol efflux by LXR ligands is mediated by ABCA1. Normal or Tangier transformed human skin fibroblasts were plated in 24-well plates and incubated for 24 h in medium A supplemented with [3H]cholesterol in the absence or presence of the indicated oxysterol (1 μg/ml) or LG268 (50 nM). ApoAI-dependent cholesterol efflux to the medium was determined as described in Materials and Methods. Data are presented as a percentage (±SE) of the total radioactivity in cells and medium; each point is the numerical average of triplicate experiments. (Inset) Quantitation of Northern blot analysis of ABCA1 mRNA levels in normal and Tangier fibroblasts normalized for GAPDH expression.
To demonstrate that the ability of the LXR signaling pathway to regulate cholesterol efflux was mediated at least in part by ABCA1, we used primary skin fibroblasts from a patient with Tangier disease that carry a loss of function mutation in the ABCA1 gene (see Materials and Methods). Induction of ABCA1 mRNA by oxysterols was similar in both cells (Fig. 5B Inset). As shown in Fig. 5B, cholesterol efflux to extracellular apoAI from wild-type human fibroblasts was strongly stimulated by treatment with 22(R)-OHC but not 22(S)-OHC. The combination of 22(R)-OHC and the RXR ligand LG268 had a greater than additive effect. By contrast, the ability of 22(R)-OHC to stimulate efflux was dramatically reduced in Tangier fibroblasts. These results provide genetic evidence that stimulation of cellular cholesterol efflux by LXR and its oxysterol ligands is mediated predominantly by the ABCA1 pathway.
Discussion
The LXRs were originally cloned as orphan receptors based on their homology to RXR (23). LXRα is expressed primarily in liver, kidney, intestine, and certain macrophages, whereas LXRβ is widely expressed (31). The physiologic ligands for both receptors are now recognized to be specific oxysterols (27–29, 32). Previous studies have delineated an important role for LXRα in the regulation of cholesterol homeostasis. LXRα null mice fail to induce transcription of the gene encoding cholesterol 7α-hydroxylase (Cyp7a), the rate-limiting enzyme in bile acid synthesis, in response to dietary cholesterol (31, 33). In addition, evidence suggests that the gene encoding cholesteryl ester transfer protein (CETP) is also regulated by LXR (34). We have recently shown that both LXRα and LXRβ are expressed in macrophages (ref. 7; P.T. and R. M. Evans, unpublished data) and that the induction of ABCG1 in response to macrophage lipid loading is mediated by LXR (7). These observations led us to explore a functional role for the LXRs in macrophage lipid metabolism.
Previous work established that expression of ABCA1 is dramatically induced in lipid-loaded macrophages and that this protein plays an important role in facilitating cellular cholesterol efflux (6, 14, 15). However, the nature of the molecular sensor that links lipid loading to the induction of ABCA1 expression and the stimulation of cholesterol efflux has remained obscure. We have shown here that activation of the nuclear receptor LXRα by oxysterol ligands leads to the transcriptional induction of ABCA1 in macrophages and fibroblasts. Moreover, retroviral expression and activation of LXRα was shown to be sufficient to promote cellular cholesterol efflux to apoAI. The ability of oxysterol ligands of LXR to stimulate cholesterol efflux was dramatically reduced in cells that lack functional ABCA1. This study represents the first demonstration that expression of ABCA1 and the process of cellular cholesterol efflux are controlled, at least in part, by a nuclear receptor–signaling pathway.
Additional studies will be required to better define the specific roles of ABCA1 and ABCG1 in regulating lipid efflux from cells. Consistent with its proposed role in regulating lipid efflux, indirect evidence suggests that the ABCA1 protein is expressed at the cell surface (16). However, it has yet to be established whether ABCA1 functions directly to transport phospholipid and/or cholesterol across the plasma membrane to exogenous lipid acceptors. Alternatively, ABCA1 might act as a phospholipid flippase and thus control lipid asymmetry. Such asymmetry could drastically affect lipid desorption/efflux to exogenous lipid acceptor proteins such as apoAI.
The role of ABCG1 in such lipid movement is even less well understood. ABCG1 encodes a half transporter protein that has been reported to be localized to both the plasma membrane and to perinuclear membranes (6). However, we have observed that an ABCG1-green fluorescent protein fusion localized to perinuclear membranes and was absent from the plasma membrane (A.V. and P.A.E., unpublished data). These latter results are consistent with many studies that demonstrate that mammalian half transporter proteins are localized to intracellular membranes, including peroxisomes, mitochondria, and endoplasmic reticulum and are excluded from the plasma membrane (35, 36). Interestingly, the small residual induction of cholesterol efflux by oxysterols observed in Tangier cells (Fig. 5B) leaves open the possibility that additional proteins or pathways may be involved, perhaps including ABCG1. It is also possible that ABCG1 acts upstream of ABCA1 in the efflux pathway. Additional studies are underway to define both the cellular location of ABCG1 and its potential role in modulating cholesterol efflux.
The demonstration that ABCA1 and ABCG1 expression is highly induced in macrophages in response to oxysterol-activated LXR is consistent with an important role for these proteins in macrophage biology, especially during lipid-loading. Such lipid-loaded macrophages are thought to influence the etiology of atherosclerosis (4, 37). Our results suggest a novel mechanism whereby cholesterol, through its conversion to oxysterol ligands of LXR, can regulate sterol efflux from cells (Fig. 6). This model suggests that the LXR pathway may be a useful target for the pharmacological regulation of cellular lipid metabolism. It is possible that increased expression of ABCA1 and/or ABCG1 in macrophages may limit the accumulation of lipid and thus protect against the development of fatty lesions and atherosclerosis. Clearly, in vivo studies and the development of high affinity synthetic ligands for LXR will be required to test this possibility.
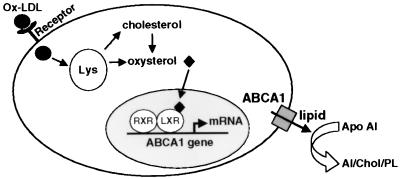
Figure 6.
Model for the regulation of ABCA1 expression and cellular cholesterol efflux by LXR in response to lipid loading. Binding of Ox-LDL to cell surface receptors (CD36, SR-A, SR-BI, etc.) leads to internalization and degradation in lysosomes (Lys). Oxysterols, either present in Ox-LDL or produced intracellularly from cholesterol, enter the nucleus and activate the LXR/RXR heterodimer on target genes such as ABCA1. Induction of ABCA1 protein expression results in increased efflux of cholesterol and/or phospholipids to apoAI.
Acknowledgments
We thank Drs. S. E. Antonarakis for human ABCG1 cDNA, R. Lawn for human ABCA1 cDNA, R. Davis for murine ABCA1 cDNA, D. Mangelsdorf for LXRα and β cDNAs, and R. Heyman for LG268. Normal and Tangier fibroblasts were a gift of Dr. John F. Oram. We thank Drs. Oram, Rothblat, and Fielding for useful discussions, Ms. Rosa Chen for typing, and Drs. J. Berliner and A. Lusis for a critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (HL 30568 to P.A.E.), the Laubisch Fund (to P.A.E.), and the Johnsson Comprehensive Cancer Center (to P.T.). P.T. is an Assistant Investigator of the Howard Hughes Medical Institute at the University of California, Los Angeles.
Abbreviations
- ABC
ATP-binding cassette
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- apoAI
apolipoprotein AI
- Ac-LDL
acetylated LDL
- Ox-LDL
highly oxidized LDL
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- OHC
hydroxycholesterol
- RXR
retinoid X receptor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200367697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200367697
References
- 1.Yamada Y, Doi T, Hamakubo T, Kodama T. Cell Mol Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown M S, Goldstein J L, Krieger M, Ho Y K, Anderson R G W. J Cell Biol. 1979;82:597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerrity R G. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 5.Langmann T, Klucken J, Reil M, Liebisch G, Luciani M-F, Chimini G, Kaminski W, Schmitz G. Biochem Biophys Res Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 6.Klucken J, Buchler C, Orso E, Kaminski W E, Porsch-Ozcurumez M, Liebisch G, Kapinsky M, Diederich W, Drobnik W, Dean M, et al. Proc Natl Acad Sci USA. 2000;97:817–822. doi: 10.1073/pnas.97.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkateswaran A, Repa J J, Lobaccaro J-M A, Bronson A, Mangelsdorf D J, Edwards P A. J Biol Chem. 2000;275:14700–14707. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- 8.Childs S, Ling V. In: Important Advances in Oncology. DeVita V T, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1994. pp. 21–36. [Google Scholar]
- 9.Dean M, Allikmets R. Curr Opin Genet Dev. 1995;5:779–785. doi: 10.1016/0959-437x(95)80011-s. [DOI] [PubMed] [Google Scholar]
- 10.Higgins C F. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 11.Luciani M F, Denizot F, Savary S, Mattei M G, Chimini G. Genomics. 1994;21:150–159. doi: 10.1006/geno.1994.1237. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Rossier C, Lalioti M D, Lynn A, Chakravarti A, Perrin G, Antonarakis S E. Am J Hum Genet. 1996;59:66–75. [PMC free article] [PubMed] [Google Scholar]
- 13.Savary S, Denizot F, Luciani M-F, Mattei M-G, Chimini G. Mamm Genome. 1996;7:673–676. doi: 10.1007/s003359900203. [DOI] [PubMed] [Google Scholar]
- 14.McNeish J, Aiello R J, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe K L, Roach M L, Royer L J, de Wet J, et al. Proc Natl Acad Sci USA. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assmann G, von Eckardstein A, Brewer H B., Jr . In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, editors. New York: McGraw-Hill; 1995. pp. 2053–2072. [Google Scholar]
- 16.Lawn R M, Wade D P, Garvin M R, Wang X, Schwartz K, Porter J G, Seilhamer J J, Vaughan A M, Oram J F. J Clin Invest. 1999;104:R25–R31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks-Wilson A, Marcil M, Clee S M, Zhang L-H, Roomp K, van Dam M, Yu L, Brewer C, Collins J A, Molhuizen H O F, et al. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 18.Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Özcürümez M, et al. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 19.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette J C, Deleuze J F, Brewer H B, Duverger N, Denefle P, et al. Nat Genet. 1999;22(4):352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 20.Orsó E, Broccardo C, Kaminski W E, Böttcher A, Liebisch G, Drobnik W, Götz A, Chambenoit O, Diederich W, Langmann T, et al. Nat Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Reyes N, Halbert C L, Smith P P, Benditt E P, McDougall J K. Proc Natl Acad Sci USA. 1992;89:1224–1228. doi: 10.1073/pnas.89.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tontonoz P, Hu E, Spiegelman B M. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 23.Willy P J, Umesono K, Ong E S, Evans R M, Heyman R A, Mangelsdorf D J. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 24.Morgenstern J P, Land H. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis G A, Knopp R H, Oram J F. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. Nature (London) 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 28.Janowski B A, Grogan M J, Jones S A, Wisely G B, Kliewer S A, Corey E J, Mangelsdorf D J. Proc Natl Acad Sci USA. 1999;96:266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, et al. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 30.Willy P J, Mangelsdorf D J. Genes Dev. 1997;11:289–298. doi: 10.1101/gad.11.3.289. [DOI] [PubMed] [Google Scholar]
- 31.Peet D J, Janowski B A, Mangelsdorf D J. Curr Opin Genet Dev. 1998;8:571–575. doi: 10.1016/s0959-437x(98)80013-0. [DOI] [PubMed] [Google Scholar]
- 32.Forman B M, Ruan B, Chen J, Schroepfer G J, Jr, Evans R M. Proc Natl Acad Sci USA. 1997;94:10588–10593. doi: 10.1073/pnas.94.20.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peet D J, Turley S D, Ma W, Janowski B A, Lobaccaro J M, Hammer R E, Mangelsdorf D J. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y, Tall A R. J Clin Invest. 2000;105:513–520. doi: 10.1172/JCI8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein I, Sarkadi B, Váradi A. Biochim Biophys Acta. 1999;1461:237–262. doi: 10.1016/s0005-2736(99)00161-3. [DOI] [PubMed] [Google Scholar]
- 36.Bauer B E, Wolfger H, Kuchler K. Biochim Biophys Acta. 1999;1461:2217–2236. doi: 10.1016/s0005-2736(99)00160-1. [DOI] [PubMed] [Google Scholar]
- 37.Navab M, Berliner J A, Watson A D, Hama S Y, Territo M C, Lusis A J, Shih D M, Van Lenten B J, Frank J S, Demer L L, et al. Arterioscler Thromb Vasc Biol. 1996;17:831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]