Abstract
BACKGROUND—Insulin-like growth factors (IGF) I and II improve myocardial function after coronary occlusion in different animal models. OBJECTIVES—To investigate the mechanism of improved myocardial function after administration of IGF-I or IGF-II in acute myocardial infarction. METHODS—Female pigs (mean (SD) weight 25 (5) kg) were subjected to acute myocardial infarction by microembolisation with 75-150 µm affigel blue beads. The beads contained and slowly released 150 µg/pig of IGF-I (n = 6), IGF-II (n = 6), or pig albumin (n = 6). Echocardiography, perfusion imaging, and haemodynamic measurements were performed before infarction and during four weeks after infarction. Regional wall motion of different left ventricular segments was scored semiquantitatively on the basis of a three point scoring system, from normal = 0 to dyskinesia = 3. Serum cardiac troponin I concentration was measured before, immediately after, and three hours after the infarct. Excised hearts were analysed for actin, desmin, blood vessel density, and DNA laddering within the infarct, border, and normal myocardial areas. RESULTS—Myocardial function of the infarct related area improved significantly during the four weeks of follow up in both the IGF groups (p = 0.01). Myocardial perfusion, heart rate, and blood pressure were similar in all the animals during the study. Treated animals had lower serum cardiac troponin I concentration (p = 0.001), more actin in the border area (p = 0.01) and infarct area (p = 0.0001), and reduced DNA laddering in the infarct area compared with the controls (p < 0.05). IGF groups had more blood vessels in the border area (p = 0.04) and the infarct area (p = 0.003). CONCLUSIONS—Both types of IGF improved myocardial function and the improvement was associated with preservation of myocardial structure. IGF-I was more effective than IGF-II. Keywords: myocardial infarction; growth factors; ventricular function; troponin I
Full Text
The Full Text of this article is available as a PDF (277.6 KB).
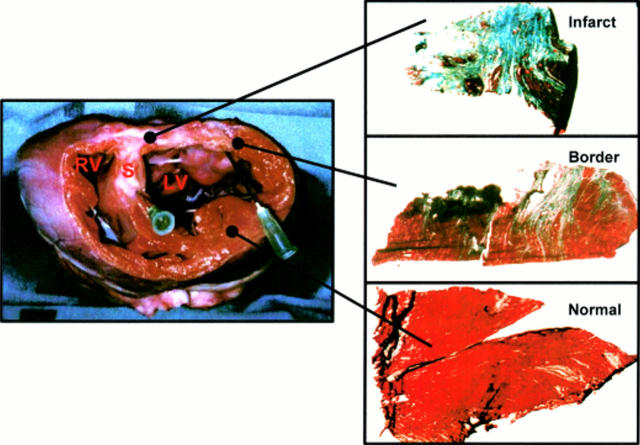
Figure 1 .
Example of macroscopic (left panel) and microscopic (right panel) examination. Infarct, border, and normal myocardium stained with Masson-trichrome: viable myocardium stains red, collagen stains blue (magnification ×200). Macroscopic picture shows the left ventricle (LV), right ventricle (RV), and septum (S). White area of the left ventricle represents scar tissue from which the "infarct" tissue sample was taken. The "normal" tissue sample was taken from the contralateral wall, and the "border" tissue sample from the area bridging the scar and the normal myocardium.
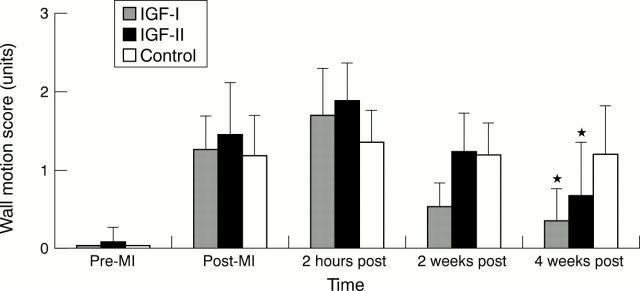
Figure 2 .
Comparison of myocardial wall motion abnormality in IGF-I, IGF-II, and control groups during the four week follow up period. Pre-MI, baseline; post-MI, immediately after myocardial infarction; 2 hours post, two hours after infarction; 2 weeks post, two weeks after infarction; 4 weeks post, four weeks after infarction. *Significant difference from control group, p = 0.01.
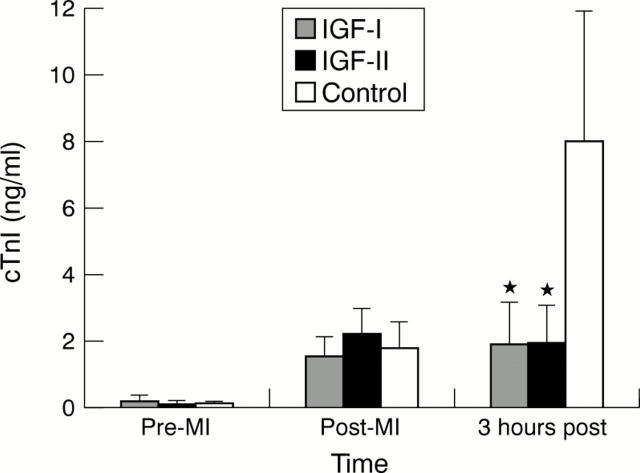
Figure 3 .
Comparison of serum cardiac troponin I (cTnI) concentrations in IGF-I, IGF-II, and control groups at baseline (pre-MI), immediately after myocardial infarction (post-MI), and three hours after infarction (3 hours post-MI). *Significant difference from control, p = 0.001.
Figure 4 .
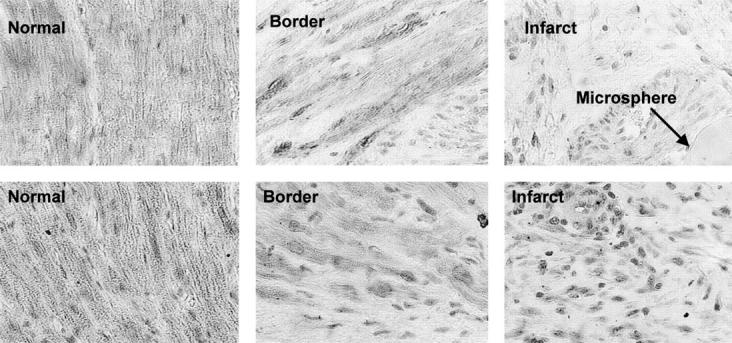
Immunostaining with monoclonal mouse IgG2a α-actin (upper panel) and monoclonal mouse IgG1 κ α-desmin (lower panel) of normal, border, and infarct areas. Lower staining intensity is observed within infarcted and border myocardial areas than in the normal area. A typical affigel blue bead microsphere is also shown.
Figure 5 .
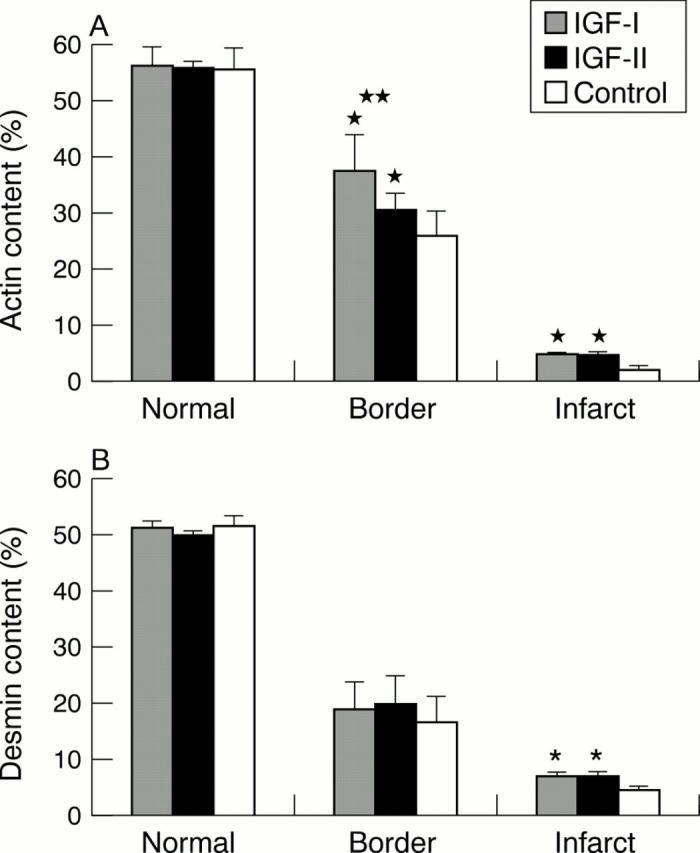
(A) Comparison of the actin content in the normal, border, and infarct myocardial areas of IGF-I, IGF-II, and control groups. *Significant difference from control group in the border area (p = 0.01) and the infarct area (p = 0.0001). **Significant difference between IGF-I and IGF-II groups in the border area (p = 0.03). (B) Comparison of desmin content in the normal, border, and infarct areas of IGF-I, IGF-II, and control groups. *Significant difference from control group in the infarct area (p = 0.0002).
Figure 6 .
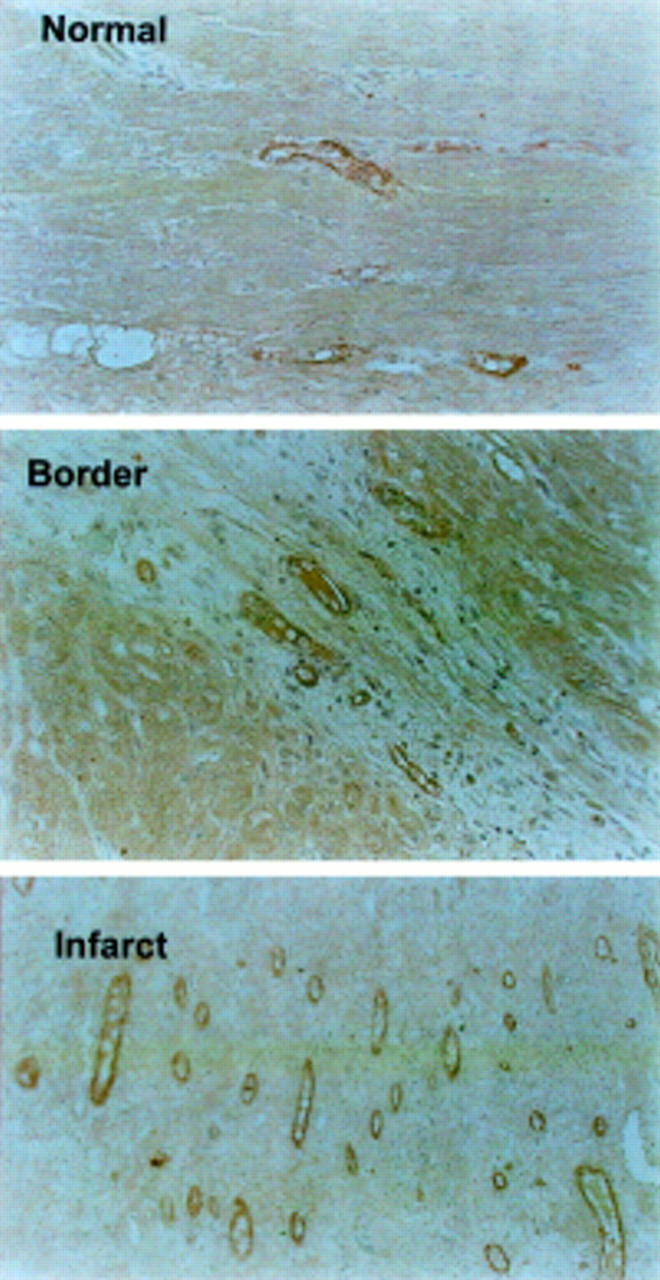
Immunostaining with factor VIII (Von Willebrand factor, VWF) antibody of normal, border, and infarct areas. Larger numbers of positively stained endothelial cells are seen within the infarcted myocardial area than in the border and normal areas.
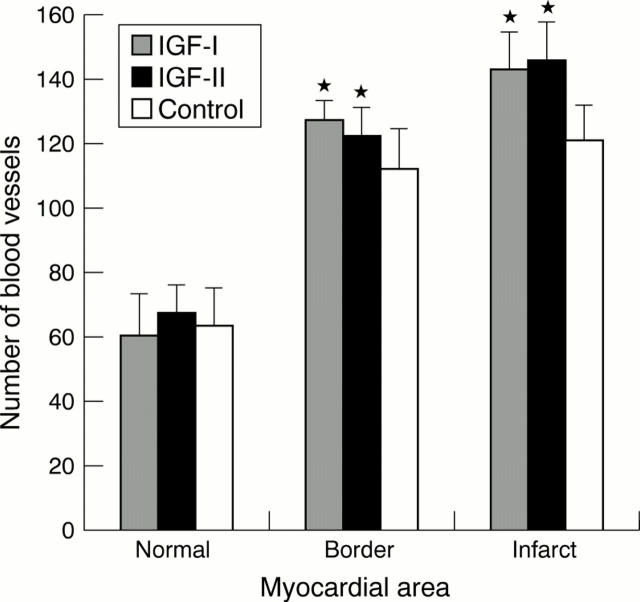
Figure 7 .
Comparison of blood vessel density in the normal, border, and infarct myocardial areas of IGF-I treated, IGF-II treated, and control groups. *Significant difference from control group in the border (p = 0.04) and infarct areas (p = 0.003).
Figure 8 .
Gel electrophoresis of DNA strand breaks (a-f) using ligation mediated polymerase chain reaction. Lanes a, b, and c represent normal, border, and infarct areas, respectively, from a representative control animal. Lanes d, e, and f represent normal, border, and infarct areas, respectively, from a representative IGF-I treated animal. The right lane represents a 123 base pair ladder of double stranded DNA (D-5042, Sigma).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi Y., Liu W., Zebrowski B., Xie K., Ellis L. M. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res. 1998 Sep 1;58(17):4008–4014. [PubMed] [Google Scholar]
- Ambler G. R., Johnston B. M., Maxwell L., Gavin J. B., Gluckman P. D. Improvement of doxorubicin induced cardiomyopathy in rats treated with insulin-like growth factor I. Cardiovasc Res. 1993 Jul;27(7):1368–1373. doi: 10.1093/cvr/27.7.1368. [DOI] [PubMed] [Google Scholar]
- Baer R. W., Payne B. D., Verrier E. D., Vlahakes G. J., Molodowitch D., Uhlig P. N., Hoffman J. I. Increased number of myocardial blood flow measurements with radionuclide-labeled microspheres. Am J Physiol. 1984 Mar;246(3 Pt 2):H418–H434. doi: 10.1152/ajpheart.1984.246.3.H418. [DOI] [PubMed] [Google Scholar]
- Battler A., Hasdai D., Goldberg I., Ohad D., Di Segni E., Bor A., Varda-Bloom N., Vered Z., Kornowski R., Lake M. Exogenous insulin-like growth factor II enhances post-infarction regional myocardial function in swine. Eur Heart J. 1995 Dec;16(12):1851–1859. doi: 10.1093/oxfordjournals.eurheartj.a060839. [DOI] [PubMed] [Google Scholar]
- Battler A., Scheinowitz M., Bor A., Hasdai D., Vered Z., Di Segni E., Varda-Bloom N., Nass D., Engelberg S., Eldar M. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol. 1993 Dec;22(7):2001–2006. doi: 10.1016/0735-1097(93)90790-8. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Evan G. I., Schwartz S. M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995 May;95(5):2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisi G., Podio V., Valetto M. R., Broglio F., Bertuccio G., DEl Rio G., Boghen M. F., Berti F., Muller E. E., Ghigo E. Radionuclide angiocardiographic evaluation of the cardiovascular effects of recombinant human IGF-I in normal adults. Eur J Endocrinol. 1999 Apr;140(4):322–327. doi: 10.1530/eje.0.1400322. [DOI] [PubMed] [Google Scholar]
- Buerke M., Murohara T., Skurk C., Nuss C., Tomaselli K., Lefer A. M. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):8031–8035. doi: 10.1073/pnas.92.17.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao A., Cuocolo A., Di Somma C., Cerbone G., Morte A. M., Pivonello R., Nicolai E., Salvatore M., Lombardi G. Does the age of onset of growth hormone deficiency affect cardiac performance? A radionuclide angiography study. Clin Endocrinol (Oxf) 2000 Apr;52(4):447–455. doi: 10.1046/j.1365-2265.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Davidson B., Goldberg I., Kopolovic J. Angiogenesis in uterine cervical intraepithelial neoplasia and squamous cell carcinoma: an immunohistochemical study. Int J Gynecol Pathol. 1997 Oct;16(4):335–338. doi: 10.1097/00004347-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Donath M. Y., Sütsch G., Yan X. W., Piva B., Brunner H. P., Glatz Y., Zapf J., Follath F., Froesch E. R., Kiowski W. Acute cardiovascular effects of insulin-like growth factor I in patients with chronic heart failure. J Clin Endocrinol Metab. 1998 Sep;83(9):3177–3183. doi: 10.1210/jcem.83.9.5122. [DOI] [PubMed] [Google Scholar]
- Duerr R. L., Huang S., Miraliakbar H. R., Clark R., Chien K. R., Ross J., Jr Insulin-like growth factor-1 enhances ventricular hypertrophy and function during the onset of experimental cardiac failure. J Clin Invest. 1995 Feb;95(2):619–627. doi: 10.1172/JCI117706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann G. L., Campbell S. E., Rakusan K. Immediate postnatal rat heart development modified by abdominal aortic banding: analysis of gene expression. Mol Cell Biochem. 1996 Oct-Nov;163-164:47–56. doi: 10.1007/BF00408640. [DOI] [PubMed] [Google Scholar]
- Fuller S. J., Mynett J. R., Sugden P. H. Stimulation of cardiac protein synthesis by insulin-like growth factors. Biochem J. 1992 Feb 15;282(Pt 1):85–90. doi: 10.1042/bj2820085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani M., Ottani F., Ferrini D., Ladenson J. H., Destro A., Baccos D., Rusticali F., Jaffe A. S. Prognostic influence of elevated values of cardiac troponin I in patients with unstable angina. Circulation. 1997 Apr 15;95(8):2053–2059. doi: 10.1161/01.cir.95.8.2053. [DOI] [PubMed] [Google Scholar]
- Gavin J. B., Maxwell L., Edgar S. G. Microvascular involvement in cardiac pathology. J Mol Cell Cardiol. 1998 Dec;30(12):2531–2540. doi: 10.1006/jmcc.1998.0824. [DOI] [PubMed] [Google Scholar]
- Hamm C. W., Goldmann B. U., Heeschen C., Kreymann G., Berger J., Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med. 1997 Dec 4;337(23):1648–1653. doi: 10.1056/NEJM199712043372302. [DOI] [PubMed] [Google Scholar]
- Harrington E. A., Bennett M. R., Fanidi A., Evan G. I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994 Jul 15;13(14):3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Hiroe M., Hirata Y., Tsujino M., Adachi S., Shichiri M., Koike A., Nogami A., Marumo F. Insulin-like growth factor-I induces hypertrophy with enhanced expression of muscle specific genes in cultured rat cardiomyocytes. Circulation. 1993 May;87(5):1715–1721. doi: 10.1161/01.cir.87.5.1715. [DOI] [PubMed] [Google Scholar]
- Kim K. W., Bae S. K., Lee O. H., Bae M. H., Lee M. J., Park B. C. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998 Jan 15;58(2):348–351. [PubMed] [Google Scholar]
- Kinugawa S., Tsutsui H., Ide T., Nakamura R., Arimura K., Egashira K., Takeshita A. Positive inotropic effect of insulin-like growth factor-1 on normal and failing cardiac myocytes. Cardiovasc Res. 1999 Jul;43(1):157–164. doi: 10.1016/s0008-6363(99)00058-9. [DOI] [PubMed] [Google Scholar]
- Kluge A., Zimmermann R., Münkel B., Mohri M., Sack S., Schaper J., Schaper W. Insulin-like growth factor I is involved in inflammation linked angiogenic processes after microembolisation in porcine heart. Cardiovasc Res. 1995 Mar;29(3):407–415. [PubMed] [Google Scholar]
- Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997 Feb 27;336(9):633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- Lee W. L., Chen J. W., Ting C. T., Ishiwata T., Lin S. J., Korc M., Wang P. H. Insulin-like growth factor I improves cardiovascular function and suppresses apoptosis of cardiomyocytes in dilated cardiomyopathy. Endocrinology. 1999 Oct;140(10):4831–4840. doi: 10.1210/endo.140.10.7082. [DOI] [PubMed] [Google Scholar]
- Li B., Setoguchi M., Wang X., Andreoli A. M., Leri A., Malhotra A., Kajstura J., Anversa P. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ Res. 1999 May 14;84(9):1007–1019. doi: 10.1161/01.res.84.9.1007. [DOI] [PubMed] [Google Scholar]
- Li Q., Li B., Wang X., Leri A., Jana K. P., Liu Y., Kajstura J., Baserga R., Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997 Oct 15;100(8):1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Nguyen M., Shing Y., Folkman J. Quantitation of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane. Microvasc Res. 1994 Jan;47(1):31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- Redaelli G., Malhotra A., Li B., Li P., Sonnenblick E. H., Hofmann P. A., Anversa P. Effects of constitutive overexpression of insulin-like growth factor-1 on the mechanical characteristics and molecular properties of ventricular myocytes. Circ Res. 1998 Mar 23;82(5):594–603. doi: 10.1161/01.res.82.5.594. [DOI] [PubMed] [Google Scholar]
- Ren J., Samson W. K., Sowers J. R. Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999 Nov;31(11):2049–2061. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- Rubin R., Baserga R. Insulin-like growth factor-I receptor. Its role in cell proliferation, apoptosis, and tumorigenicity. Lab Invest. 1995 Sep;73(3):311–331. [PubMed] [Google Scholar]
- Schiller N. B., Shah P. M., Crawford M., DeMaria A., Devereux R., Feigenbaum H., Gutgesell H., Reichek N., Sahn D., Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989 Sep-Oct;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- Schwarz E. R., Schaper J., vom Dahl J., Altehoefer C., Grohmann B., Schoendube F., Sheehan F. H., Uebis R., Buell U., Messmer B. J. Myocyte degeneration and cell death in hibernating human myocardium. J Am Coll Cardiol. 1996 Jun;27(7):1577–1585. doi: 10.1016/0735-1097(96)00059-9. [DOI] [PubMed] [Google Scholar]
- Sell C., Baserga R., Rubin R. Insulin-like growth factor I (IGF-I) and the IGF-I receptor prevent etoposide-induced apoptosis. Cancer Res. 1995 Jan 15;55(2):303–306. [PubMed] [Google Scholar]
- Serneri G. G., Modesti P. A., Boddi M., Cecioni I., Paniccia R., Coppo M., Galanti G., Simonetti I., Vanni S., Papa L. Cardiac growth factors in human hypertrophy. Relations with myocardial contractility and wall stress. Circ Res. 1999 Jul 9;85(1):57–67. doi: 10.1161/01.res.85.1.57. [DOI] [PubMed] [Google Scholar]
- Solaro R. J. Troponin I, stunning, hypertrophy, and failure of the heart. Circ Res. 1999 Jan 8;84(1):122–124. doi: 10.1161/01.res.84.1.122. [DOI] [PubMed] [Google Scholar]
- Staley K., Blaschke A. J., Chun J. Apoptotic DNA fragmentation is detected by a semi-quantitative ligation-mediated PCR of blunt DNA ends. Cell Death Differ. 1997 Jan;4(1):66–75. doi: 10.1038/sj.cdd.4400207. [DOI] [PubMed] [Google Scholar]
- Vogt A. M., Htun P., Kluge A., Zimmermann R., Schaper W. Insulin-like growth factor-II delays myocardial infarction in experimental coronary artery occlusion. Cardiovasc Res. 1997 Feb;33(2):469–477. doi: 10.1016/s0008-6363(96)00212-x. [DOI] [PubMed] [Google Scholar]