Abstract
An in vitro colony-forming assay and flow cytometry were used to identify rat hepatoblasts as being classical MHC class I, RT1Al-, OX18low intercellular adhesion molecule 1 (ICAM-1)+. Inducible differentiation toward biliary lineage was observed in most colonies derived from single RT1Al- progenitors, proving their bipotentiality. These findings demonstrate the antigenic profile of clonogenic hepatoblasts and proof of their bipotency. Furthermore, whereas colony formation of adult hepatocytes required epidermal growth factor, clonal growth of hepatoblasts was potentiated without epidermal growth factor. The adult hepatic colonies consisted of RT1Al+OX18+ICAM-1++ cells. These results indicate that hepatoblasts possess unique characteristics as compared with adult hepatocytes harboring significant proliferative activity. The phenotypic identity of hepatoblasts and the clonal culture system have relevance for identifying hepatic stem cells from adults, for studying liver development, and for cell therapy based on hepatic progenitors.
Identification of multipotential progenitor populations in mammalian tissues is important both for therapeutic potential and an understanding of developmental processes and tissue homeostasis. Progenitor populations are ideal targets for gene therapy, cell transplantation, and tissue engineering of bioartificial organs (1, 2). A demand for liver progenitors is increasing because of a severe shortage of donor organs for orthotopic liver transplantation. Because liver transplantation currently is the only successful therapy for patients suffering chronic liver failure, cell therapy with hepatic progenitors offers an alternative approach for therapies of liver diseases.
In the context of liver development, hepatic cells in the early fetal liver are designated as hepatoblasts. These are bipotent progenitors that give rise to hepatocytic and biliary lineages of cells (3, 4). As early as embryonic day (E) 13 in rat, hepatic progenitors are thought to be a homogeneous population with developmentally equal bipotentiality (4). However, direct evidence for bipotency of these progenitors has not yet been shown. Moreover, because the antigenic identity of hepatoblasts has not been determined, neither clonal identification nor purification of hepatoblasts for clonal analyses has been possible.
The mammalian adult liver has a tremendous capacity to recover after either extensive hepatotoxic injury or partial hepatectomy (5). Data from recent studies in the mouse have been interpreted to suggest that some medium- to large-sized (21–27 μm) hepatocytes from adults have an extensive growth potential as assayed by transplantation experiments (6). However, no evidence has been suggested for biliary epithelial differentiation in the transgenic mouse system (6). On the other hand, recent in vitro studies indicated that small adult hepatocytes (less than 20 μm) demonstrate higher growth potential than that of larger hepatocytes (7). Even though physiological significance of these subpopulations in normal liver turnover remains controversial, it is suggested that in vivo repopulatable hepatocytes and in vitro highly proliferative hepatocytes are different subpopulations based on the size fractionation (6, 7). Moreover, the relevance of progenitors to either in vivo or in vitro growth is uncertain, especially because it is unknown whether hepatoblasts are conserved as progenitors in adult livers.
Materials and Methods
Rats.
Pregnant Fisher 344 rats were obtained from the Charles River Breeding Laboratories. The morning on which the plug was observed was designated day 0. Male Fisher 344 rats (200–250 g) were used for adult liver cells. All animal experiments have been conducted in accordance with institutional guidelines.
Cell Preparation.
E13 fetal livers were digested with 800 units/ml collagenase (Sigma) and 20 units/ml thermolysin (Sigma) followed by further digestion with trypsin-EDTA solution (Sigma). The cell suspension was treated with 200 units/ml DNase I (Sigma). Adult liver cells were obtained by a two-step liver perfusion method as described (8). After perfusion, the cells were filtrated with a 30-μm sieve to remove large aggregated cells. Cellular viability was >90% as measured by trypan blue exclusion.
Hepatic Cell Lines and Cell Culture.
Several hepatic cell lines were established from E15 fetal liver on STO (American Type Culture Collection) feeders and in a serum-free hormonally defined medium (HDM), as described in detail in the supplemental material, which is published on the PNAS website, www.pnas.org. The HDM was developed based on other serum-free media (9–11). STO feeder cells were prepared as described (12). The cell adhesion assays for hepatic cell lines and the description of the isolation of STO sublines and STO5 cells transfected with pEF-Hlx-MC1neo [a kind gift from J. M. Adams, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia (13)] are described in the supplemental materials.
Immunohistochemical Staining of Colonies.
Culture plates were fixed in methanol-acetone (1:1) for 2 min at room temperature, rinsed, and blocked with 20% goat serum (GIBCO/BRL) at 4°C. For double labeling of α-fetoprotein (AFP) and albumin (ALB), plates were incubated with anti-rat ALB antibody (ICN) followed by Texas Red-conjugated anti-rabbit IgG (Vector Laboratories) and FITC-conjugated anti-rat AFP polyclonal antibody (Nordic, Immunological Laboratories, Tilburg, The Netherlands). For double labeling of ALB and cytokeratin (CK) 19, anti-CK19 mAb (Amersham Pharmacia) and FITC-conjugated anti-mouse IgG (Caltag, South San Francisco, CA) were used.
Flow Cytometric Analysis.
Cells stained with mAbs were analyzed on a FACScan (Becton Dickinson) or sorted by using a Moflow (Cytomation, Fort Collins, CO). After blocking with 20% goat serum and 1% teleostean gelatin (Sigma), hepatic cell lines and adult liver cells were stained with FITC-conjugated anti-RT1Aa,b,l B5 (PharMingen), anti-RT1A OX18 (PharMingen), phycoerythrin (PE)-conjugated anti-rat intercellular adhesion molecule 1 (ICAM-1) 1A29 (PharMingen) or FITC-conjugated anti-rat integrin β1 Ha2/5 (PharMingen). FITC-conjugated anti-mouse IgG was used for OX18. Cell suspensions of the hepatic cell lines or freshly isolated cells cultured on STO feeders were stained with biotinilated anti-mouse CD98 (PharMingen) followed by a second staining with streptavidin-RED670 (GIBCO/BRL) as well as anti-rat mAbs to gate out mouse STO cells. The cells from E13 fetal liver were stained with FITC-B5, PE-1A29, and biotinylated OX18 followed by streptavidin-RED670 for three-color staining.
Colony-Forming Assay (CFA) for Hepatic Cell Lines, Sorted Cells, and Adult Liver Cells.
The hepatic cell lines were plated in triplicate at 500 cells/9.6 cm2 on STO feeders and in HDM. The cultures were incubated for 10–14 days with medium changes every other day. Double immunofluorescence staining of AFP and ALB then was performed. One hundred colonies per well were analyzed in the colony morphology and for the expression of AFP and ALB. The colonies were stained by Diff-Quik (Dade, Düdingen, Switzerland) to count the number of colonies per well. As another minor modification in the CFA for freshly isolated cells, the plating cell number was changed as described in Results; the culture period was expanded to 14–17 days; the concentration of dexamethasone and epidermal growth factor (EGF) was changed to 10-6 M and 10 ng/ml respectively. For CFA of biliary differentiation, double staining of ALB and CK19 of the colonies was performed at 5-day intervals of the culture in the presence or absence of EGF. At day 5 of the cultures, a colony with more than one CK19+ cell was counted as a CK19+ colony. At days 10 and 15, a colony containing multiple clusters of two CK19+ cells or one cluster of more than three CK19+ cells was counted as a CK19+ colony. Each point represents the mean ± SD from triplicate-stained cultures. In the CFA for adult liver cells, small clumps of liver cells could not be eliminated by the regular preparation. Therefore, an undefined number of the colonies might be produced from the clumps.
Results
Establishment of a CFA for Hepatic Progenitors.
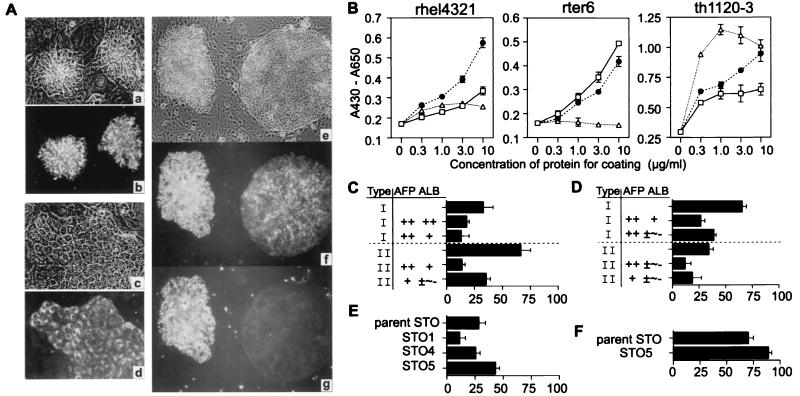
Although a number of defined culture conditions have been developed for hepatic cells (9, 11, 14), none supports the growth of liver cells at sufficiently low density to permit true clonal analysis. To develop an in vitro CFA system for identification of clonogenic hepatoblasts necessitates culture conditions that support the growth of a single cell to a colony and in which cell expansion is associated with retention of the phenotypic characteristics of the cell of origin. Hepatic cell lines were used initially as models for hepatic progenitors. Several hepatic cell lines from rat fetal livers were established on feeders of a STO embryonic cell line and in HDM. Rhel4321 consisted mostly of packed or aggregated small cells, whereas th1120–3 made only flattened monolayers (Fig. 1A a–d). Rter6 produced aggregate clusters and flattened monolayers constantly even after repetitive cloning. In addition to the morphological difference in the three cell lines, they had different adhesive affinity to specific components of extracellular matrices. Whereas collagen type IV was the most effective in the attachment of th1120–3, similar to the findings for the adult liver cells (15), it worked less well for rter6 and rhel4321 (Fig. 1B). Laminin proved the most effective substratum for adhesion of rhel4321 (Fig. 1B). The preference to laminin rather than fibronectin or collagen type IV in rhel4321 is similar to that of fetal hepatic cells (15). Using these cell lines, a systematic CFA was developed. When rter6 was cultured on the feeders at a seeding density of 52 cells/cm2, the cell line produced two types of colonies. In Fig. 1Ae, the left colony consisted of aggregated small cells (type I colony), whereas the other colony at the right produced a flattened monolayer (type II colony). Double immunostaining of AFP and ALB, phenotypic markers for early hepatic progenitors, indicated that intense expression of both AFP and ALB was observed only in type I colonies (Fig. 1A f and g). These results indicate that this culture condition can support the growth of a single cell to a colony maintaining the phenotypic characteristics of early hepatic progenitors. Furthermore, STO subclones were compared in their ability to support aggregated colonies because almost all type I colonies of rter6 and rhel4321 strongly expressed AFP, whereas type II colonies of cells did not (Fig. 1 C and D). One of the clones, STO5, supported the colony formation more than any of the other sublines and more than the parent line (Fig. 1 E and F). Therefore, STO5 was used for clonal analysis of primary hepatic cells, because the culture conditions yielding the highest production of type I colonies were assured to be the best for clonal assays for freshly isolated hepatic progenitors.
Figure 1.
Comparative characterization of hepatic cell lines. (A) Phase contrast and the immunofluorescence micrograph of rhel4321 (a and b), th1120–6 (c and d), and rter6 (e–g) are presented. Rhel4321 (b) and th1120–6 (d) were labeled with anti-AFP. Rter6 was doubly labeled with Abs for AFP (f) and ALB (g). Original magnifications: a–d, ×200; e–g, ×100. (B) Cell adhesion assay. Each cell line was cultured in microtiter wells coated with laminin (●), fibronectin (□), or collagen IV (▵). The number of adherent cells was measured. CFA of rter6 (C and E) and rhel4321 (D and F). The degrees of AFP and ALB expression were divided into four ranges: ++ (90–100% of cells are positive), + (10–90%), ± (1–10%), and − (0%). Blank column represents only the types of the colonies. x axis indicates colony number (C and D) or aggregated colony number (E and F) per 100 colonies. Data shown represent the means ± SD of triplicates.
Surface Antigenic Profiles of the Hepatic Cell Lines.
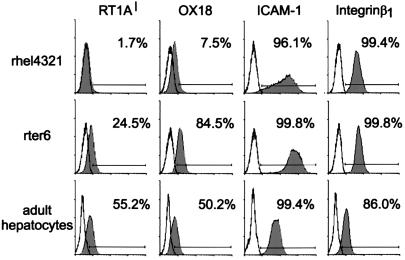
The antigenic profiles of the two hepatic cell lines and freshly isolated adult liver cells were characterized. Compared with adult hepatocytes and rter6, the antigenic profile of the rhel4321 is quite unique in that there is no expression of RT1Al, rat classical MHC class I in the Fisher 344 strain, and fairly low signals identifiable by mAb OX18, which recognizes nonpolymorphic MHC class I antigens (Fig. 2). The pattern of the ICAM-1 expression in rhel4321 is heterogeneous, whereas integrin β1 was expressed uniformly in the line.
Figure 2.
Surface phenotypes of hepatic cell lines (rhel4321 and rter6) and adult hepatocytes. They were stained with mAbs anti-RT1Al, OX18, anti-ICAM-1, or anti-integrin β1 (closed histogram). The cell number is plotted versus log10 of the mean fluorescence intensity. The solid line indicates the unstained cells or secondary antibody alone.
Clonal Identification of Hepatic Progenitors in Fetal Livers.
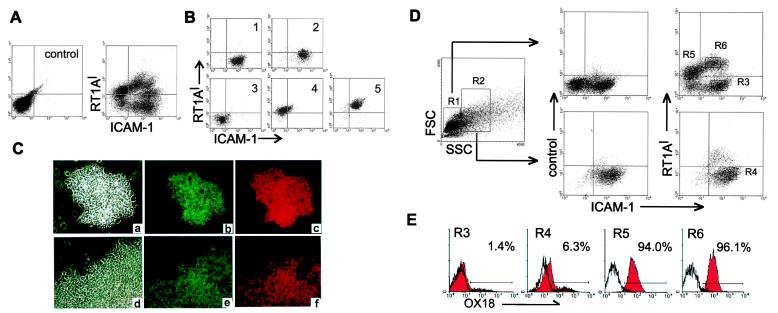
Hepatogenesis and massive amounts of hematopoiesis coexist in the fetal liver. Because adult bone marrow cells other than mature erythrocytes strongly express MHC class I molecules (data not shown), it was expected that the fetal hepatic population could be separated effectively from the hematopoietic cell populations by MHC class I expression. The cell suspensions from rat E13 livers were stained with MHC class I, ICAM-1, and integrin β1 mAbs. The two-color staining pattern of RT1Al and ICAM-1 showed several populations (Fig. 3A), whereas integrin β1 was expressed uniformly in most of the cells (data not shown). To determine which population contained the hepatic cells, five fractions were isolated by flow cytometric sorts and then screened by the CFA. Fig. 3B represents the result of resorting of the five sorted fractions (gates 1–5). The hepatic cell colonies, defined by expression of ALB and AFP, were also distinguishable morphologically (Fig. 3C), enabling one to count the number of hepatic colonies per well. The majority of the hepatic colonies were detected in gate 2 (Table 1). A small number of the colonies appeared in gate 1. The other fractions contained negligible numbers of cells with hepatic colony-forming ability. Evidently the culture conditions supported clonal expansion of hepatic colonies without loss of the expression of AFP and ALB (Fig. 3C). To investigate the MHC class I expression on the hepatic cells in detail, three-color staining of RT1Al, ICAM-1, and OX18 with the side scatter (SSC), a reflection of the granularity of cell, as another parameter, were used for the cell fractionation (R1 and R2). Fig. 3D shows that the cells in gate 2 (Fig. 3B) derived from the R2 gate exclusively. Gating R2 based on the SSC, the population corresponding to the gate 2, clearly showed RT1Al- and OX18low phenotype (Fig. 3 D and E). The CFA of these cells confirmed that R4, but not R3, harbors colony-forming cells (Table 1). These results suggest that the RT1Al-OX18lowICAM-1+ population from E13 rat liver consists of hepatic progenitors.
Figure 3.
Phenotypic analysis of E13 rat fetal livers. (A) Cells were stained with anti-RT1Al and anti-ICAM-1 mAbs. Unstained cell suspension is indicated for negative control. (B) After cell sorting, the collected cells of five fractions (1–5 on the upper left column) based on the expression of RT1Al and ICAM-1 were resorted. Each fractionated cell population was cultured for the CFA (Table 1). (C) Immunofluorescence labeling of hepatic colonies with Abs for AFP (b and e) and ALB (c and f) are shown with corresponding phase contrast fields (a and d). d–f represent a part of a large colony. Original magnification: ×200. (D) Cells were stained with anti-RT1Al, anti-ICAM-1, and anti-OX18. Two gates (R1 and R2) were created with respect to differences in side scatter. The cells included in each gate were analyzed with respect to their expression of RT1Al and ICAM-1. The histogram (E) represents the expression of OX18 in each gate (R3–R6) of D.
Table 1.
The frequency of hepatic colonies from sorted fetal liver cells
| Experiment | Inoculated cell
number
|
Hepatic colony number
|
Colony
efficiency, %
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | |
| Gate 1 | 500 | 500 | 500 | 19 | 28 | 8.7 | 3.8 | 5.7 | 1.7 |
| Gate 2 | 500 | 500 | 500 | 88 | 99 | 136 | 17.7 | 19.9 | 27.3 |
| Gate 3 | 500 | 5,000 | 5,000 | 0.7 | 8 | 10 | 0.1 | 0.2 | 0.2 |
| Gate 4 | 500 | 5,000 | 5,000 | 0 | 2.3 | 6.3 | 0 | 0.1 | 0.1 |
| Gate 5 | 500 | 5,000 | 5,000 | 0.3 | 1.7 | 5 | 0.1 | 0 | 0.1 |
| IV | V | VI | IV | V | VI | IV | V | VI | |
| R3 | 500 | 500 | 1,000 | 1 | 3.7 | 7 | 0.2 | 0.7 | 0.7 |
| R4 | 500 | 500 | 500 | 176 | 167 | 269 | 35.2 | 33.4 | 53.9 |
Flow cytometric sorted cells from each fraction in Fig. 3 were cultured on feeders at indicated cell number per well (9.6 cm2). The hepatic colony number (per well) was given from triplicate-stained cultures (mean). Colony efficiency is expressed as the percentage of cells inoculated in culture that went on to form colonies after 16 days of the culture. Results of six independent experiments are shown.
Evidence for Bipotentiality in the Clonogenic Hepatic Progenitors.
At E13 of gestation in the rat, the hepatic cells have a developmental potential, giving rise to mature hepatocytes and bile duct epithelium, even though they express no markers for the biliary lineage (4). If the identified hepatic progenitors are bipotent, the fate of biliary lineage could be dictated under distinct culture conditions. To test the hypothesis, the sorted RT1Al-OX18lowICAM-1+ cells were cultured in the presence or absence of EGF followed by the double immunostaining of CK19 and ALB for the colonies to monitor their fates at 5-day intervals.
CK19 is one of the most remarkable markers for biliary epithelial cells in adult liver, because hepatocytes don't express CK19 at all (16). However, CK19 can't be detected during hepatic development before E15 in the rat fetus (4). As development progresses, maturing bile ducts gradually lose the expression of ALB (4). Therefore, the induction of the expression of CK19 and the disappearance of the ALB expression can be used as the indicator for initial development of a biliary phenotype.
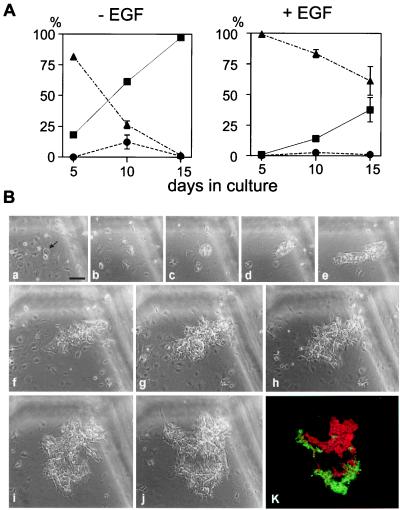
After the first 5 days, the CK19+ colonies were negligible in the cultures treated with EGF, whereas a few colonies containing CK19+ cells occurred in the absence of EGF (Fig. 4A). Although the intensity of the CK19 expression was fairly weak, the CK19+ cells showed reduced ALB expression. At the 10th day of the culture, some of the colonies contained only CK19+ cells or ALB+ cells, and others had both of them (Fig. 4A). In the majority of colonies in the presence of EGF, CK19 expression was not induced at the 10th day of the culture. In the absence of EGF, most of the colonies contained CK19+ cells by day 15 of culture (Fig. 4A), although the number of CK19+ cells in a single colony varied among the colonies. The pattern of the CK19+ and ALB+ cells in a single colony, as shown in Fig. 4B, was reciprocal. Some of the colonies, however, consisted of cells coexpressing ALB and CK19 (data not shown). Furthermore, the results of single-cell analysis designed by single-cell culture (Table 2) and serial observation of colony formation from single cells followed by staining for ALB and CK19 (Fig. 4B) demonstrated the clonogenic bipotent nature of the isolated RT1Al- cells. Collectively, the RT1Al-OX18lowICAM-1+ cells from E13 fetal liver are bipotent hepatic progenitors, hepatoblasts, with heterogeneous response to inductive cues for biliary lineage.
Figure 4.
Bipotency of differentiation of multiparametric sorted hepatic progenitors. (A) Cells were fixed at 5-day intervals after starting the culture in the presence or absence of EGF and stained for ALB and CK19, and the proportion of the types of colonies was determined. ■, ▴, and ● represent the colonies containing ALB+ cells and CK19+ cells, only ALB+ cells, and only CK19+ cells, respectively. Data shown represent the means ± SD of triplicates. (B) The bipotent nature of a single RT1Al- hepatic progenitor. The hepatic progenitors were cultured at 52 cells/cm2 in the absence of EGF. A single cell was selected as shown by an arrow and was monitored for colony formation. Scratches at the top and the right in each field were made on the bottom surface of the culture plate by a needle. Phase contrast images of the culture at 24 h (a), day 2 (b), day 3 (c), day 4 (d), day 6 (e), day 7 (f), day 8 (g), day 9 (h), day 11 (i), and day 12 (j) after plating are shown. After 12 days of culture, the colony was double-stained for ALB and CK19. The merged immunofluorescence image (k) of ALB (red) and CK19 (green) corresponds to that shown in phase contrast (j). All magnifications are the same as a. (Bar = 100 μm.)
Table 2.
Single-cell culture analysis of RT1Al− hepatic progenitors
| Experiment | Culture system | Inoculated cell no. per well | Total no. of wells for inoculation | Identified colony no. | Colony efficiency, % | Proportion of ALB+ CK19+ colonies, % |
|---|---|---|---|---|---|---|
| I | Single-cell culture | 1 | 192 | 61 | 32 | 92 |
| CFA | 420 | 3 | 394* | 31 | 96 | |
| II | Single-cell culture | 1 | 192 | 31 | 16 | 97 |
| CFA | 500 | 3 | 220* | 15 | 95 |
Sorted hepatic progenitor cells were cultured on feeders in four 48-well plates at one cell per well in the absence of EGF. A standard CFA was performed for a comparative analysis. After 15 days of the culture, hepatic colonies were doubly stained for ALB and CK19. All colonies contained ALB+ cells, and the proportion of colonies also containing CK19+ cells among the hepatic colonies are indicated above. The total number of the colonies was counted after visible dye staining.
Colony number of CFA was combined from triplicate-stained cultures.
Comparative Analysis of Hepatoblasts and Highly Proliferative Hepatocytes.
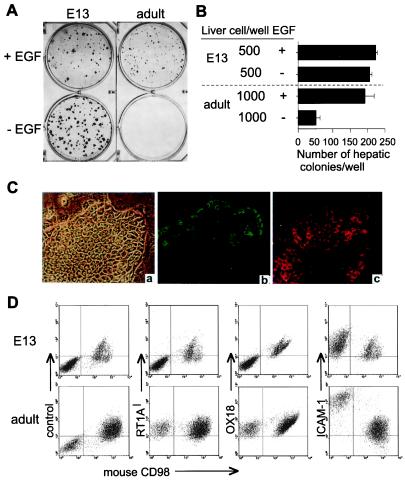
It was unknown whether or not highly proliferative hepatocytes in adult liver conserve any antigenic or characteristic phenotypes of hepatoblasts in early fetal liver. The colony formation, morphology, and ex vivo growth requirements as well as proliferative capability of sorted hepatoblasts were compared with that of colonies of hepatocytes from adult liver by using the defined feeders and the HDM. As shown in Fig. 5A, the colony size of the hepatoblasts increased in the absence of EGF, whereas adult hepatocytes yielded large colonies only in the presence of EGF (Fig. 5B). The morphology of the colonies derived from adult liver cells was the flattened monolayer with the modest expression of AFP and ALB (Fig. 5C). After over 2 weeks of culture, the expression of RT1Al, OX18, and ICAM-1 was assessed. As shown in Fig. 5D, the expression of RT1Al, OX18, and ICAM-1 did not change in the culture of hepatoblasts. On the other hand, the expression of RT1Al and OX18 in the highly proliferative hepatocytes from adult liver was clearly positive. Whereas the antigenic profiles of either freshly isolated or cultured hepatocytes in adults is RT1Al+OX18+ICAM-1+, the expression of ICAM-1 was induced intensively in colony-forming cells in culture relative to that of freshly isolated cells (Figs. 2 and 5D). EGF did not affect the expression of MHC class I expression (data not shown). Furthermore, the average cell number in a single colony was calculated from the recovered cell number, the percentage of rat hepatic cells, and the colony efficiency. The estimated cell number in colonies from hepatoblasts reached 3–4 × 103 cells, whereas that from adult hepatocytes was 130 cells on average, indicating less growth potential under the conditions used (Table 3, which is published as supplemental material).
Figure 5.
Comparative analysis of hepatoblasts and highly proliferative hepatocytes. (A) Hepatoblasts from E13 liver or adult liver cells were cultured on STO feeders for 16 days then stained with Diff-Quik. (B) The colony number in the culture with or without EGF. The number of the hepatic colonies per well was counted from triplicate cultures. Data shown represent the means ± SD of triplicates. (C) Hepatic colony from adult liver (a–c). The micrographs of the phase contrast (a) and the double labeling with anti-AFP (b) and anti-ALB (c) are shown. Original magnification: ×200. (D) The surface analysis of cultured hepatoblasts and adult liver cells. Cultured cells were stained with mAbs, anti-RT1Al, OX18, or anti-ICAM-1. Mouse CD98 negative population represent rat cells.
Discussion
The antigenic profile of hepatoblasts in E13 rat liver has been found to be RT1Al-OX18lowICAM-1+. Because no other cellular subpopulation showed such significant numbers of hepatic colonies in the assay, it is suggested that this antigenic profile is that for the earliest cells in the rat hepatic lineage. So far, all hepatic cells as early as E13 in the rat are thought to be a homogeneous population and able to differentiate to biliary epithelial cell lineage as well as to mature hepatocytes. A clonal culture system established in this study allowed the growth of hepatic cells at sufficiently low density and high efficiency of colony formation to perform definitive clonal analysis for specific cellular subpopulations in the fetal liver. The data in which most colonies from single RT1Al-OX18lowICAM-1+ cells contained some descendents having a biliary phenotype supports the hypothesis. The clonal assay, however, clearly showed that the response to inductive cues for biliary lineage is different in subpopulations of hepatic progenitors. Some of them quickly lose the expression of ALB and begin CK19 expression (Fig. 4A). In addition, the clonal culture conditions were able to modify their fates. The stromal factors from STO cells in the absence or presence of EGF are good candidates for eliciting specific fate determination, because it is speculated that the mesenchymal factors from the connective tissue surrounding the portal vein are important for biliary differentiation (17).
Furthermore, the culture system based on STO feeders and HDM detected a subpopulation of adult liver cells that are highly proliferative even at clonal densities. There are three remarkable differences between hepatoblasts and these highly proliferative hepatocytes. First, the hepatocytes express RT1Al (Fig. 5C). Second, the hepatocytes produce flattened colonies, whereas colonies from hepatoblasts are aggregates consisting of small cells during the primary expansion (Figs. 3C and 5C). Third, the growth of the highly proliferative hepatocytes is dramatically reduced in the absence of EGF, whereas the clonal growth of hepatoblasts is potentiated in the absence of EGF (Fig. 5 A and B). Moreover, the magnitude of ex vivo proliferation capability is more potent in hepatoblasts under the clonal culture conditions (Table 3). Although neither RT1Al- cells nor aggregated colonies were observed with or without EGF in adult liver cell cultures, it remains to be determined whether or not counterparts of hepatoblasts exist in the adult liver given the method of isolation or cultivation of the adult cells.
Laboratory rats other than those with the RT1c haplotype possess only one functional classical MHC class I locus (18). Therefore, the data indicating an absence of surface expression of RT1Al on R4 cell fraction (Fig. 3D) mean that rat hepatoblasts do not express classical MHC class I on the cell surface. There are typically two groups of MHC class I antigens, classical MHC class I and nonclassical MHC class I (MHC class Ib). Classical MHC class I performs a critical role in immune surveillance, according to present exogenous antigens at the cell surface to antigen-specific αβ T cell receptors on CD8 T lymphocytes, whereas most of MHC class Ib have yet to be assigned a function. The weak positive signal by OX18 on the hepatoblasts (Fig. 3E) suggests that they express certain MHC class Ib antigens, e.g., mouse Q10, the expression pattern of which is similar to that of serum proteins such as ALB and AFP (19). Furthermore, it is well documented that undifferentiated pluripotent teratocartinomas tightly suppress the expression of classical MHC class I but do not suppress a few of the MHC class Ib genes (20). The expression of classical MHC class I is induced after differentiation (21). Therefore, classical MHC class I expression is one of the developmentally controlled antigens. This is the case in the hepatic lineage also. Here, the developmental heterogeneity has been established clearly in the hepatic lineage.
As classical MHC class I genes are highly polymorphic (22), they also provoke a strong allograft response. The highly proliferative hepatocytes after ex vivo expansion induced an intense expression of ICAM-1 expression in addition to the MHC class I expression, whereas hepatoblasts retained the original phenotype (Fig. 5D). Peptide-MHC complex and ICAM-1 antigens are crucial molecules for the formation of the “immunological synapse,” a specialized junction between a T cell and a target cell (23). Therefore, ex vivo expanded hepatoblasts and hepatocytes should be different in the context of immunological recognition by CD8 T cells. The unique phenotype of undetectable expression of classical MHC class I and lower expression of ICAM-1 on the hepatoblasts might be advantageous and be relied on for the escape from the host immune system when they are transplanted in an MHC incompatible host.
Further characterization of identified clonogenic hepatoblasts and use of the CFA system will be important for clinical therapies based on hepatic progenitors. Interestingly, a previous immunohistochemical study suggests that the developing human hepatic cells in fetuses express only MHC class Ib antigens, but not classical MHC class I antigens (24). If the human hepatic progenitors have a similar unique phenotype to that of rat hepatoblasts in terms of no classical MHC class I and modest ICAM-1 expression, are bipotential with respect to biliary and hepatocytic lineages, and have potent growth capability, this progenitor population should have great promise for nonautologous cell transplantation, ex vivo gene therapy, or bioartificial liver devices.
Supplementary Material
Acknowledgments
We thank J. M. Adams for plasmids, A. Fisher for operating the cell sorter, Y.-W. Rong for liver perfusions, N. Moss for helpful comments on the manuscript, and C. Lodestro for laboratory management. This work was supported by grants from the National Institutes of Health (RO1-DK52851) and Renaissance Cell Technologies, Research Triangle Park, NC. The Advanced Cell Technologies and Tissue Engineering Core of the Center for Gastrointestinal and Biliary Disease Biology (National Institutes of Health Grant DK34987; R. Sandler) provided assistance with respect to adult liver perfusions.
Abbreviations
- CFA
colony-forming assay
- E(n)
embryonic day
- AFP
α-fetoprotein
- ALB
albumin
- CK
cytokeratin
- HDM
hormonally defined medium
- ICAM-1
intercellular adhesion molecule 1
- EGF
epidermal growth factor
References
- 1.Weissman I L. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 2.Xu A S L, Luntz T L, MacDonald J M, Kubota H, Hsu E, London R E, Reid L M. In: Principles of Tissue Engineering. Lanza R P, Langer R, Chick W L, editors. San Diego: Academic; 2000. pp. 559–598. [Google Scholar]
- 3.Douarin N M. Med Biol. 1975;53:427–455. [PubMed] [Google Scholar]
- 4.Shiojiri N, Lemire J M, Fausto N. Cancer Res. 1991;51:2611–2620. [PubMed] [Google Scholar]
- 5.Higgins G M, Anderson R M. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 6.Overturf K, Al-Dhalimy M, Finegold M, Grompe M. Am J Pathol. 1999;155:2135–2143. doi: 10.1016/S0002-9440(10)65531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tateno C, Takai-Kajihara K, Yamasaki C, Sato H, Yoshizato K. Hepatology. 2000;31:65–74. doi: 10.1002/hep.510310113. [DOI] [PubMed] [Google Scholar]
- 8.Seglen P O. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 9.Gatmaitan Z, Jefferson D M, Ruiz-Opazo N, Biempica L, Arias I M, Dudas G, Leinwand L A, Reid L M. J Cell Biol. 1983;97:1179–1190. doi: 10.1083/jcb.97.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chessebeuf M, Padieu P. In Vitro. 1984;20:780–795. doi: 10.1007/BF02618294. [DOI] [PubMed] [Google Scholar]
- 11.Mitaka T, Sattler C A, Sattler G L, Sargent L M, Pitot H C. Hepatology. 1991;13:21–30. [PubMed] [Google Scholar]
- 12.Robertson E J. In: Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. Robertson E J, editor. Oxford: IRS; 1987. pp. 71–112. [Google Scholar]
- 13.Allen J D, Adams J M. Blood. 1993;81:3242–3251. [PubMed] [Google Scholar]
- 14.Block G D, Locker J, Bowen W C, Petersen B E, Katyal S, Strom S C, Riley T, Howard T A, Michalopoulos G K. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirata K, Yoshida Y, Shiramatsu K, Freeman A E, Hayasaka H. Exp Cell Biol. 1983;51:121–129. doi: 10.1159/000163182. [DOI] [PubMed] [Google Scholar]
- 16.Moll R, Franke W W, Schiller D L, Geiger B, Krepler R. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 17.Roskams T, Van Eyken P, Desmet V. In: Liver Growth and Repair. Strain A J, Diehl A M, editors. London: Chapman & Hall; 1998. pp. 541–557. [Google Scholar]
- 18.Joly E, Leong L, Coadwell W J, Clarkson C, Butcher G W. J Immunol. 1996;157:1551–1558. [PubMed] [Google Scholar]
- 19.David-Watine B, Transy C, Gachelin G, Kourilsky P. Gene. 1987;61:145–154. doi: 10.1016/0378-1119(87)90109-0. [DOI] [PubMed] [Google Scholar]
- 20.Ostrand-Rosenberg S, Nickerson D A, Clements V K, Garcia E P, Lamouse-Smith E, Hood L, Stroynowski I. Proc Natl Acad Sci USA. 1989;86:5084–5088. doi: 10.1073/pnas.86.13.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowles B B, Pan S, Solter D, Linnenbach A, Croce C, Huebner K. Nature (London) 1980;288:615–618. doi: 10.1038/288615a0. [DOI] [PubMed] [Google Scholar]
- 22.Bjorkman P J, Parham P. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- 23.Grakoui A, Bromley S K, Sumen C, Davis M M, Shaw A S, Allen P M, Dustin M L. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 24.Houlihan J M, Biro P A, Fergar-Payne A, Simpson K L, Holmes C H. J Immunol. 1992;149:668–675. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.