Abstract
Understanding the ecological mechanisms that underlie extinction is fundamental to conservation. It is well established that not all taxa are equally vulnerable to extinction, but the reasons for these differences are poorly understood. This may be, in part, because different taxa are threatened by different mechanisms. Theoretically, sources of extinction risk that perturb the balance between fecundity and longevity, such as human persecution and introduced predators, should be particularly hazardous for taxa that have slow rates of population growth. In contrast, sources of extinction risk that reduce niche availability, such as habitat loss, should represent a particular threat to taxa that are ecologically specialized. Here we test these predictions by using a phylogenetic comparative method and a database on 95 families of birds. As theory predicts, extinction risk incurred through persecution and introduced predators is associated with large body size and long generation time but is not associated with degree of specialization, whereas extinction risk incurred through habitat loss is associated with habitat specialization and small body size but not with generation time. These results demonstrate the importance of considering separately the multiple mechanisms that underlie contemporary patterns of extinction. They also reveal why it has previously proven so difficult to identify simple ecological correlates of overall extinction risk.
Keywords: life history, body size
Extinction risk is not evenly distributed among avian lineages (1–5). Some families, such as the parrots (Psittacidae), rails (Rallidae), cranes (Gruidae), pheasants (Phasianidae), pigeons (Columbidae), and albatrosses and petrels (Procellariidae) contain significantly more threatened species than predicted by chance alone, whereas other families, such as the woodpeckers (Picidae), contain far fewer species than expected (1, 3). We have only a poor understanding of why some lineages are threatened whereas others appear secure (6, 7). Several proximate correlates of extinction risk have been identified, such as small population size or restricted geographic range (8–11), but it has proven more difficult to establish which fundamental ecological parameters incur an elevated extinction risk (1, 6, 7, 12). One reason for this may be that different taxa are put at risk of extinction through different ecological mechanisms (8, 9, 13). For instance, theory predicts that sources of extinction risk that act through perturbing the balance between fecundity and longevity, such as human persecution and introduced predators, should be particularly hazardous for taxa that have slow rates of population growth (1, 2, 8, 9, 13–17). On the other hand, sources of extinction risk that reduce niche availability, such as habitat loss, should be most dangerous to species that are ecologically specialized (13–15, 18). These contrasting predictions have been explored at a local level (8, 9, 13, 14), but they have not been tested in a systematic way across higher taxa or across geopolitical boundaries. Large-scale analyses to date have been based on overall measures of extinction risk, measures that are composite across all sources of threat (1–5, 11, 12, 19). If the theory is correct, such composite measures may mask underlying mechanisms. We should expect that different lineages are vulnerable to different sources of extinction risk, and that different ecological factors are associated with each source of extinction risk.
The overall aim of this study was to test the predictions that (i) different taxa are prone to different mechanisms of extinction, and (ii) different ecological factors are associated with different mechanisms of extinction. We emphasize, however, that we do not aim to provide an exhaustive study of all possible sources of extinction risk or all plausible ecological factors. Rather, we study the two most important sources of extinction risk for birds—habitat loss and human persecution/introduced predators (13, 20)—versus three illustrative ecological factors: body size, residual generation time (after controlling for variation in body size), and degree of habitat specialization. Our analyses aim to provide a framework for future study and illustrate why that framework is important. They do not identify all ecological mechanisms of extinction in birds.
Methods
Database.
We collated data on 95 avian families with respect to extinction risk and ecology. Our measure of extinction risk was the proportion of species in a family that currently was listed as being threatened by extinction (20). Unlike previous analyses (1–4, 12), we partitioned extinction risk according to the source of threat: habitat loss, human persecution and/or introduced predators, introduced competitors, hybridization, disease, and unknown risk factors (20). Thus, each threatened species in ref. 20 was categorized as being either threatened by habitat loss or introduced predators/direct persecution on the basis of the species-specific accounts in that source. We could not use the raw International Union for Conservation of Nature and Natural Resources (IUCN) codes, because, although they did identify extinction risk incurred via persecution leading to rapid decline in population size (IUCN code A1c), they did not identify the role of persecution in leading to small range or small population size (IUCN codes B, C, and D). Nor did they distinguish between different sorts of introduced species (predators/competitors/disease, etc.) (IUCN code A1d) or identify habitat loss per se as the cause of declines, small population sizes, or small range sizes (IUCN codes A, B, C, and D). All of this information was, however, available in the individual species reports (20). Habitat loss included changes in agricultural practice and water management as well as deforestation. Direct human persecution included sustenance harvesting, poisoning, egg collecting, and trade. The scoring of extinction risk was done by a third party (S. Scott, University of Queensland, Brisbane, Australia) and thus was performed blind with respect to the ecological data and hypotheses being tested.
Our three ecological variables were body size, residual generation time, and degree of habitat specialization. All three of these factors previously have been invoked to explain variation between bird species in extinction risk (1, 2, 8–10, 12–19), and all were previously used by us in a family-level analysis of species richness in birds (21). Family typical body size was measured in g by using female-specific weights wherever possible (21). Family-typical generation time was measured as modal age at first breeding among females in months (21). Because modal age at first breeding in birds is known to be significantly positively correlated with body size (22), we calculated residual generation time. Residual generation time was calculated by taking the standardized residuals, resulting from a linear regression model of generation time on log (body size)[model fitted to raw family-typical data: r = 0.533, n = 95, P < 0.0001, regression slope = 9.50 (SE ± 1.58)]. Family-typical degree of habitat specialization was measured on a three-point scale on the basis of the number of habitat categories used by a species during the breeding season: 2 points, only one habitat category; 1 point, uses two habitat categories; and 0 points, uses three or more habitat categories (21). Habitat categories were saltwater or estuarine, freshwater, forest, and open (21). Data were collected from as many species in each family as possible (21). Family-typical values were the modal value across species. This database is available from the authors on request.
Comparative Analyses.
The first question we tackled was whether different taxa are threatened by the same sources of extinction risk. Here, we calculated separately the proportion of threatened species in each family that was threatened by (i) habitat loss alone, (ii) human persecution/introduced predators alone, (iii) both habitat loss and human persecution/introduced predators, and (iv) other sources of threat. We then used regression models to test for an association between the proportion of a family that is threatened by habitat loss alone versus the proportion of a family that is threatened by human persecution/introduced predators alone. Because the data on proportion of a species that is threatened by extinction was not normally distributed, we used square root transformations before model fitting (23). Also, because many families (34 in our database) contained no threatened species at all, we fitted two sets of models: the first set was based on all 95 families in our database, and the second set excluded those families that did not contain any threatened species.
The second question we addressed was whether extinction risk via habitat loss and extinction risk via human persecution/introduced predators was correlated with different ecological factors. Here, we tested for associations between the variation among families in these two types of extinction risk and variation among families in our three candidate explanatory ecological variables, such as body size, residual generation time, and degree of habitat specialization.
For each of these two questions, we performed our analyses in two stages: initially, we performed analyses by using families as independent data points, and then subsequently we repeated all analyses by using evolutionarily independent contrasts (24, 25). In the case of our analyses of the ecological correlates of extinction risk based on families as independent data points, all three ecological factors were treated as binary variables. Body size was (small) 1–1,000 g and (large) 1,000–100,000 g. Residual generation time was (short) modal age at first breeding less than predicted from overall allometric relationship between age at first breeding and body size (i.e., negative residual value), and (long) modal age at first breeding older than predicted from allometric relationship (i.e., positive residual). Habitat specialization was (generalist) more than one breeding habitat, and (specialist) only one breeding habitat. One-way ANOVAs then were used to test whether variation between the categories of the ecological variables was associated with variation in each source of extinction risk.
In the case of our analyses of the ecological correlates of extinction risk based on evolutionarily independent contrasts, we sought to maximize the number of contrasts while allowing the same sort of analysis to be applied to all ecological factors. Thus, all ecological variables were treated as categorical variables. Body size was ranked on a five-point scale: 0 points, 1–100 g; 1 point, 100–1,000 g; 2 points, 1,000–10,000 g; 3 points, 10,000–100,000; and 4 points, 100,000–1,000,000 g. Generation time was residual modal age at first breeding, where residual values were calculated from regression models based on independent contrasts rather than on family-typical values. The raw habitat-specialization scale was used as a rank scale. In each analysis, the ecological variable was treated as the main variable. For each ecological variable, we performed two tests: first, a pair of sign tests to establish whether changes in the ecological variable were associated with changes in extinction risk via habitat loss and/or extinction risk via persecution/predation, respectively; and second, a χ2 test to test for an association between the type of extinction risk and the direction of change in extinction risk. In all analyses based on contrasts, the caic software program (26), in combination with Sibley and Ahlquist's “tapestry” phylogeny (27), was used to identify and calculate evolutionarily independent contrasts (24–26). Branch lengths were set according to DNA–DNA hybridization temperatures (27). When addressing our first question, the association between the proportion of families threatened via habitat loss versus the proportion of families threatened by persecution/predation, we used the crunch algorithm in caic because both variables were continuous, and fitted regressions through the origin (26). When addressing our second question, whether variation in ecology was associated with variation in each type of extinction risk, we used the brunch algorithm in caic because the ecological variable was categorical (26).
Results
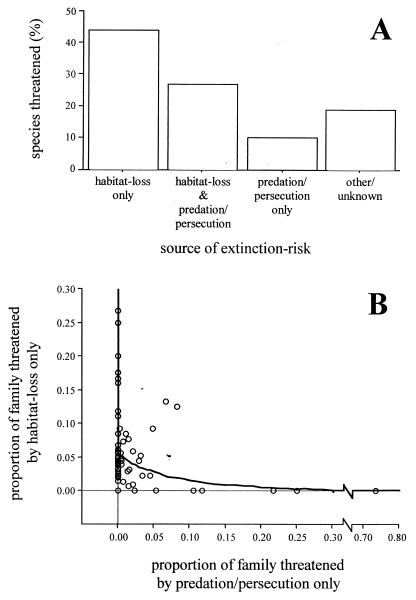
Among the 1,012-threatened species in our database, habitat loss and human persecution/introduced predators were by far the most common threats. Habitat loss was cited as a source of risk for over 70% of threatened species, whereas human persecution and/or introduced predators were cited in 35% of cases. Overall, therefore, twice as many species (54%) were classified as being threatened by either habitat loss alone or human persecution/introduced predators alone than being threatened by both sources together (27%) (Fig. 1A).
Figure 1.
Sources of extinction risk among threatened birds. (A) Relative proportion of species in database threatened by each source of extinction. Data are individual threatened species. “Other” sources of extinction risk include competition, hybridization, and disease. (B) Association between proportion of species in each family threatened by habitat loss only versus proportion of species in each family threatened by persecution/introduced predators only; the heavy black line shows the results of regression model restricted to only those families that contain threatened species (61 families: F = 23.35, df = 1, 59, P < 0.0001; (proportion of family threatened by habitat loss only) = [0.42–0.49 × (proportion of family threatened by persecution/predation only)1/2]2. Data are raw family-typical values.
With respect to our first question, we found that when we included all 95 families in our database, there was no significant correlation between the proportion of a family that is threatened by habitat loss alone and the proportion threatened through human persecution/introduced predators alone (linear regression model on square-root transformed data: r = 0.08, n = 95, P = 0.45). However, when we restricted our analyses to just those families that contained threatened species, there was a significant negative correlation between the proportion of a family threatened via habitat loss and the proportion of family threatened via persecution/predation (Fig. 1B; r = 0.53, n = 61, P < 0.0001). Moreover, both of these results remained the same when we based our analyses on independent contrasts rather than family-typical values (model based on contrasts across all families; r = 0.01, n = 94, P = 0.92: model based on contrasts after nonthreatened families were excluded; r = 0.45, n = 60, P = 0.004). Such patterns showed that among birds, different sources of extinction risk affect different lineages to varying extents. They also raise the possibility that the major sources of extinction risk are mutually exclusive to one another. This in turn suggested that different sources of extinction risk may have different ecological correlates.
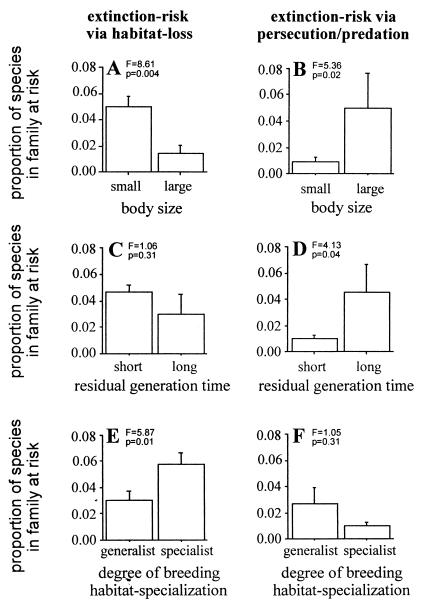
With respect to our second question, we found consistent and robust associations between variation in extinction risk and variation in both candidate ecological factors (Fig. 2). The patterns of association were, however, strikingly different between the two sources of extinction risk. Extinction risk incurred through habitat loss was associated with small body size (Fig. 2A) and a high degree of habitat specialization (Fig. 2E), but was not significantly associated with residual generation time (Fig. 2C). Extinction risk incurred through human persecution and/or introduced predators, on the other hand, was associated with large body size (Fig. 2B) and long residual generation time (Fig. 2D), but was not associated with variation in the degree of habitat specialization (Fig. 2F). Furthermore, these results remained largely unchanged when we controlled for differing degrees of shared ancestry among the families by repeating our analyses on evolutionarily independent contrasts. Here, increases in body size were associated with significant increases in extinction risk via predation/persecution (12/15 changes, sign test P = 0.03), but no consistent directional change in extinction risk via habitat loss (11/19 negative changes, P = 0.64). This represents a significant difference between the two sources of extinction risk (χ2 = 6.38, df = 2, P = 0.03). Similarly, increases in generation time were associated with significant increases in extinction risk via predation/persecution (15/24 changes, P = 0.013), but not with consistent directional changes and decreases in extinction risk via predation/persecution (13/21 positive changes, P = 0.38). Again, this represents a significant difference between the two sources of extinction risk (χ2 = 4.18, df = 2, P = 0.04). Finally, increases in degree of habitat specialization were associated with significant increases in extinction risk via habitat loss (19/26 changes, P = 0.04) and significant increases in extinction risk via predation/persecution (12/18 changes, P = 0.05). This does not represent a significant difference between the two sources of extinction risk (χ2 = 0.01, df = 2, P = 0.91).
Figure 2.
Associations between ecology and extinction risk across avian families, with separate analyses for extinction risk via habitat loss versus extinction risk via human persecution/introduced predators. Body size versus extinction risk via (A) habitat loss and (B) persecution/predation. Residual generation time (controlling for variation in body size) versus extinction risk via (C) habitat loss and (D) persecution/predation. Degree of breeding habitat specialization versus extinction risk incurred via (E) habitat loss and (F) persecution/predation. For body size, (small) families in which modal body size is less than or equal to 1,000 g, and (large) a modal body size of over 1,000 g. For generation time, (short) families in which the modal age at first breeding is younger than expected from allometric relationship between age at first breeding and body size, and (long) an age at first breeding older than expected. For breeding habitat specialization, (specialist) families in which species typically use only one type of breeding habitat category, and (generalist) families in which typically species use more than one type of breeding habitat. On the vertical axis of each graph, the proportion of each family threatened by extinction risk is the proportion of species in that family classified as being threatened by extinction via the appropriate source of threat. All analyses are based on raw family-typical values for 95 avian families. Error bars show SEM; statistics show results of one-way ANOVAs. Degrees of freedom in all ANOVAs = 1, 93.
All of the results based on analysis of independent contrasts were qualitatively the same if all branch lengths were set to the same length. All conclusions even remained largely the same when we used nontransformed data on the proportion of each family threatened, different indices to categorize generation time and habitat specialization, raw generation time instead of residual generation time, or examined other fundamental ecological variables such as clutch size and degree of feeding specialization (unpublished data). This shows that our results are robust to the specific phylogenetic hypothesis used to calculate the evolutionarily independent contrasts, the exact form of statistical analysis used, and the precise ecological variables tested.
Discussion
Our analyses support the predictions that different lineages are threatened by different mechanisms of extinction, and that different ecological factors predispose taxa to different sources of extinction risk. The two sources of extinction risk that we investigated, habitat loss and human persecution/introduced predators, were by far the most important sources of extinction risk in our database, affecting 70% and 35% of species, respectively (13, 20). However, it was relatively unusual for a species to be threatened by both these sources of extinction risk. Twice as many species (54%) were classified as being threatened by either habitat loss alone or human persecution/introduced predators alone than being threatened by both sources together (27%). Indeed, when we looked at these patterns at the family level, we found that, if we restricted our analyses to those families that actually contained threatened species, there was a significant negative correlation between the proportion of species in a family that are threatened by habitat loss and the proportion of species that are threatened by human persecution/introduced predators. These results suggest that different lineages are vulnerable to different mechanisms of extinction, with lineages that are highly threatened by one source usually being relatively secure with respect to the other source. Such results point strongly to the possibility that different ecological factors will be associated with different sources of extinction risk.
When we tested for associations between variation in our three ecological variables and variation in extinction risk, we did indeed find very different patterns for each of the two sources of extinction risk. Whereas extinction risk via habitat loss was positively correlated with the degree of habitat specialization and small body size but not significantly associated with residual generation time, extinction risk incurred via human persecution and/or introduced predators was correlated with large body size and slow life history but was not significantly associated with variation in ecological specialization. These results confirm the prediction that different ecological factors are responsible for making a lineage vulnerable to different sources of extinction (8, 9, 13). Thus, it may be unwise to attempt to understand mechanisms of extinction based on overall composite indices of extinction risk. Such indices are likely to mask the diversity of ecological mechanisms that lead to extinction among contemporary species.
Our results also reveal two general explanations for the puzzle that variation in overall extinction risk, although usually found to be strongly correlated with proximate demographic factors such as population size and geographic range (8–11), is often only weakly correlated with variation in theoretically plausible fundamental ecological factors (1, 6, 7). The first explanation is straightforward: Some ecological factors are associated only with particular sources of extinction threat. In the case of our analyses, the extent of habitat specialization was associated only with extinction risk incurred via habitat loss, whereas residual generation time was associated only with extinction risk via persecution/predation. The second explanation is more subtle: Some ecological factors are positively associated with one type of extinction risk but negatively associated with another type of extinction risk. In our analyses, body size was an example of this sort of factor, being positively associated with extinction risk incurred via human persecution/introduced predators but negatively associated with extinction risk via habitat loss.
We do not suggest that we have identified all factors associated with extinction risk in birds. We have simply used three well-established candidate factors to illustrate the contrasting patterns of association that occur with different sources of extinction threat. Nevertheless, our results corroborate the prediction (8, 9, 13) that there are multiple routes to extinction among birds. One route is for large-bodied, slow-breeding species to become threatened when an external factor, such as human persecution or introduced predators, disrupts the delicate fecundity-mortality balance (1, 2, 8, 9). In our database, this route applies to families such as the kiwis (Apterigidae); cassowaries (Casuariidae); megapodes (Megapodiidae); penguins (Spheniscidae); and albatrosses, petrels and allies (Procellariidae). A second route is for ecologically specialized species to become threatened by habitat loss (13, 14, 18). Such families include the hummingbirds (Trochilidae); trogons (Trogonidae); scrub-birds (Menuridae); and logrunners (Orthonychidae). Inevitably, a small number of families are prone to both sources of extinction risk. These include the parrots (Psittacidae); rails (Rallidae); pheasants and allies (Phasianidae); pigeons (Columbidae); cranes (Gruidae) and white-eyes (Zosteropidae). It is this last set of families that have previously been identified as being significantly overprone to extinction (1, 5).
Our approach of partitioning overall extinction risk according to different sources of threat has provided statistically robust correlations between fundamental ecological factors and global patterns of extinction risk. We re-emphasize, however, that here we have only used three ecological variables to illustrate the value of this approach. The next step is to use this method to test the explanatory power of further ecological factors, and in particular, look at the interactions between proximate factors like geographic range size and fundamental ecological factors such as ecological specialization. The same approach also could be extended to partitioning extinction risk according to the proximate reasons for classifying a species as being threatened by extinction: small population size, small population range, fluctuating population size, and so on (28, 29). Such approaches provide the opportunity to move beyond simply describing the patterns of extinction threat to begin to understand the evolutionary and ecological processes that led to those patterns (30).
Acknowledgments
We thank A. Purvis and A. Rambaut for giving us a copy of the caic program; S. Scott for scoring threatened species with respect to source of extinction risk; K. Arnold, S. Blomberg, L. Bromham, M. Cardillo, G. Cowlishaw, S. Clegg, D. Fisher, P. Harvey, K. Jones, J. Kikkawa, G. Mace, S. Nee, V. Olson, H. Possingham, A. Purvis, A. Rambaut, and A. Thomas for discussion, providing unpublished manuscripts, and/or for comments on the manuscript; and the Australian Research Council for support. Some of this work was done while visiting the Department of Zoology of the University of Oxford. We thank P. Harvey for sabbatical hospitality.
Abbreviation
- IUCN
International Union for Conservation of Nature and Natural Resources
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 11688.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200223397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200223397
References
- 1.Bennett P M, Owens I P F. Proc R Soc London Ser B. 1997;264:401–408. [Google Scholar]
- 2.Gaston K J, Blackburn T M. Evol Ecol. 1997;11:557–565. [Google Scholar]
- 3.Russell G J, Brooks T M, McKinney M L, Anderson C G. Conserv Biol. 1998;12:1365–1376. [Google Scholar]
- 4.McKinney M L. Anim Conserv. 1998;1:159–164. [Google Scholar]
- 5.McKinney M L, Lockwood J L. Trends Ecol Evol. 1999;14:450–453. doi: 10.1016/s0169-5347(99)01679-1. [DOI] [PubMed] [Google Scholar]
- 6.Lawton J L. In: Extinction Rates. Lawton J H, May R M, editors. Oxford: Oxford Univ. Press; 1995. pp. 147–163. [Google Scholar]
- 7.May R M. Philos Trans R Soc London B. 1999;354:1951–1959. doi: 10.1098/rstb.1999.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pimm S L, Jones H L, Diamond J. Am Nat. 1988;132:757–785. [Google Scholar]
- 9.Pimm S L. The Balance of Nature. Chicago: Univ. Chicago Press; 1991. [Google Scholar]
- 10.Gaston K J. Rarity. Oxford: Blackwell Scientific; 1994. [Google Scholar]
- 11.Stattersfield A J, Crosby M J, Long A J, Wege D C. Endemic Bird Areas of the World: Priorities for Biodiversity Conservation. Cambridge, U.K.: Birdlife International; 1998. [Google Scholar]
- 12.Gaston K J, Blackburn T M. Philos Trans R Soc London B. 1995;347:205–212. [Google Scholar]
- 13.Diamond J. In: Extinctions. Nitecki M H, editor. Chicago: Chicago Univ. Press; 1984. pp. 191–246. [Google Scholar]
- 14.Brown J H. Am Nat. 1971;105:467–478. [Google Scholar]
- 15.Brown J H, Maurer B A. Science. 1989;243:1145–1150. doi: 10.1126/science.243.4895.1145. [DOI] [PubMed] [Google Scholar]
- 16.Brown J H. Macroecology. Chicago: Univ. Chicago Press; 1995. [Google Scholar]
- 17.Maurer B A. Untangling Ecological Complexity: The Macroscopic Perspective. Chicago: Univ. Chicago Press; 1999. [Google Scholar]
- 18.Bibby C J. In: Extinction Rates. Lawton J H, May R M, editors. Oxford: Oxford Univ. Press; 1995. pp. 98–110. [Google Scholar]
- 19.Gotelli N J, Graves G R. J Biogeog. 1990;17:315–325. [Google Scholar]
- 20.Collar N J, Crosby M J, Stattersfield A J. Birds to Watch 2: The World List of Threatened Birds. Cambridge, U.K.: Birdlife International; 1994. [Google Scholar]
- 21.Owens I P F, Bennett P M, Harvey P H. Proc R Soc London Ser B. 1999;266:933–939. [Google Scholar]
- 22.Owens I P F, Bennett P M. Proc R Soc London Ser B. 1995;261:227–232. [Google Scholar]
- 23.Sokal R R, Rohlf F J. Biometry. 2nd Ed. New York: Freeman; 1981. [Google Scholar]
- 24.Felsenstein J. Am Nat. 1985;125:1–15. [Google Scholar]
- 25.Harvey P H, Pagel M. The Comparative Method in Evolutionary Biology. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 26.Purvis A, Rambaut A. Comput Appl Biosci. 1995;11:247–250. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- 27.Sibley G C, Ahlquist J E. Phylogeny and Classification of Birds. New Haven, CT: Yale Univ. Press; 1990. [Google Scholar]
- 28.McKinney M L. Annu Rev Ecol Syst. 1997;28:495–516. [Google Scholar]
- 29.Purvis, A., Gittleman, J. L., Cowlishaw, G. & Mace, G. M. (2000) Proc. R. Soc. London Ser. B, in press. [DOI] [PMC free article] [PubMed]
- 30.Bennett P M, Owens I P F, Baillie J E M. In: Biological Homogenization. Lockwood J L, McKinney M L, editors. New York: Kluwer; 2000. , in press. [Google Scholar]