Abstract
Aims—Type C gastritis caused by bile reflux has a characteristic appearance, similar to that seen in other forms of chemical gastritis, such as those associated with NSAIDs or alcohol. An increase in mucosal cell proliferation increases the likelihood of a neoplastic clone of epithelial cells emerging, particularly where there is chronic epithelial injury associated with bile reflux. It has been shown previously that type C gastritis is associated with increased cell proliferation in the postsurgical stomach. The aim of this study was to determine cell proliferation in type C gastritis caused by bile reflux affecting the intact stomach.
Methods—Specimens from 15 patients with a histological diagnosis of type C gastritis on antral biopsy were obtained from the pathology archives between 1994 and 1997. A control group of nine normal antral biopsies was also selected and all underwent MIB-1 immunostaining. The gastric glands were divided into three zones (zone 1, gastric pit; zone 2, isthmus; and zone 3, gland base) and the numbers of positively staining nuclei for 500 epithelial cell nuclei were counted in each zone to determine the percentage labelling index (LI%).
Results—Cell proliferation was significantly higher in all three zones of the gastric glands with type C gastritis compared with controls as follows: zone 1, median LI% in type C gastritis 64.7 (range, 7.8–99.2), controls 4.7 (range, 2.0–11.3); zone 2, median LI% in type C gastritis 94.7 (range, 28.8–98.7), controls 40.2 (range, 23.1–70.3); and zone 3, median LI% in type C gastritis 20.0 (range, 1.3–96.0), controls 2.6 (range, 0.9–8.7).
Conclusions—Bile reflux is thought to act as a promoter of gastric carcinogenesis in the postsurgical stomach. The same may be true in the intact stomach.
Key Words: cell proliferation • epithelial kinetics • chemical gastritis
Full Text
The Full Text of this article is available as a PDF (146.7 KB).
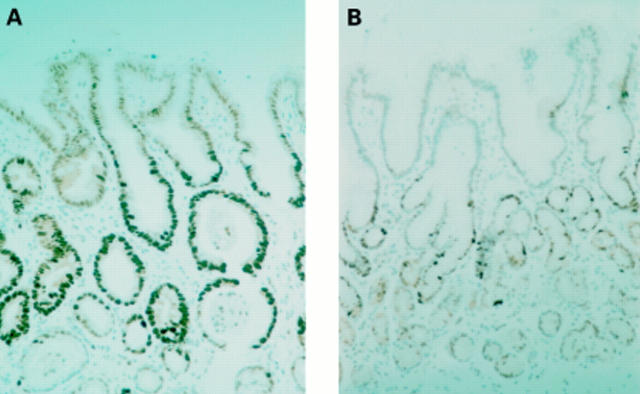
Figure 1 MIB-1 immunostaining of gastric glands in (A) type C gastritis (B) normal mucosa.
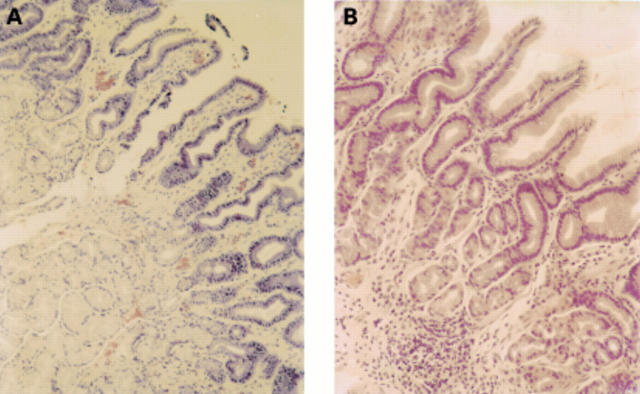
Figure 2 Haematoxylin and eosin staining of gastric glands in (A) type C gastritis (B) normal mucosa.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelman H. D. Gastritis: terminology, etiology, and clinicopathological correlations: another biased view. Hum Pathol. 1994 Oct;25(10):1006–1019. doi: 10.1016/0046-8177(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Bartram H. P., Scheppach W., Englert S., Dusel G., Richter A., Richter F., Kasper H. Effects of deoxycholic acid and butyrate on mucosal prostaglandin E2 release and cell proliferation in the human sigmoid colon. JPEN J Parenter Enteral Nutr. 1995 May-Jun;19(3):182–186. doi: 10.1177/0148607195019003182. [DOI] [PubMed] [Google Scholar]
- Bechi P., Balzi M., Becciolini A., Amorosi A., Scubla E., Giachè V., Mazzanti R., Tonelli P., Cortesini C. Gastric cell proliferation kinetics and bile reflux after partial gastrectomy. Am J Gastroenterol. 1991 Oct;86(10):1424–1432. [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992 Dec 15;52(24):6735–6740. [PubMed] [Google Scholar]
- Dixon M. F., O'Connor H. J., Axon A. T., King R. F., Johnston D. Reflux gastritis: distinct histopathological entity? J Clin Pathol. 1986 May;39(5):524–530. doi: 10.1136/jcp.39.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A. G., Sim R., Sankey E. A., Dhillon A. P., Pounder R. E. Effect of eradication of Helicobacter pylori on gastric epithelial cell proliferation. Aliment Pharmacol Ther. 1994 Apr;8(2):167–173. doi: 10.1111/j.1365-2036.1994.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Houghton P. W., Mortensen N. J., Thomas W. E., Cooper M. J., Morgan A. P., Burton P. Intragastric bile acids and histological changes in gastric mucosa. Br J Surg. 1986 May;73(5):354–356. doi: 10.1002/bjs.1800730509. [DOI] [PubMed] [Google Scholar]
- Ladas S. D., Katsogridakis J., Malamou H., Giannopoulou H., Kesse-Elia M., Raptis S. A. Helicobacter pylori may induce bile reflux: link between H pylori and bile induced injury to gastric epithelium. Gut. 1996 Jan;38(1):15–18. doi: 10.1136/gut.38.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso D., Pezzolla F., Linsalata M., Caruso M. L., Giorgio P., Guerra V., Misciagna G., Piccioli E., Di Leo A. Duodenogastric reflux, histology and cell proliferation of the gastric mucosa before and six months after cholecystectomy. Acta Gastroenterol Belg. 1995 Jan-Feb;58(1):43–50. [PubMed] [Google Scholar]
- Lynch D. A., Axon A. T. Helicobacter pylori, gastric cancer and gastric epithelial kinetics: a review. Eur J Gastroenterol Hepatol. 1995 Aug;7 (Suppl 1):S17–S23. [PubMed] [Google Scholar]
- Lynch D. A., Clarke A. M., Jackson P., Axon A. T., Dixon M. F., Quirke P. Comparison of labelling by bromodeoxyuridine, MIB-1, and proliferating cell nuclear antigen in gastric mucosal biopsy specimens. J Clin Pathol. 1994 Feb;47(2):122–125. doi: 10.1136/jcp.47.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. A., Mapstone N. P., Clarke A. M., Jackson P., Dixon M. F., Quirke P., Axon A. T. Cell proliferation in the gastric corpus in Helicobacter pylori associated gastritis and after gastric resection. Gut. 1995 Mar;36(3):351–353. doi: 10.1136/gut.36.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. A., Mapstone N. P., Clarke A. M., Sobala G. M., Jackson P., Morrison L., Dixon M. F., Quirke P., Axon A. T. Cell proliferation in Helicobacter pylori associated gastritis and the effect of eradication therapy. Gut. 1995 Mar;36(3):346–350. doi: 10.1136/gut.36.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J. D., Stratagoules E. D., LaRue J. M., Powell A. A., Gause P. R., Craven M. T., Payne C. M., Powell M. B., Gerner E. W., Earnest D. L. Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 1998;31(2):111–118. doi: 10.1080/01635589809514689. [DOI] [PubMed] [Google Scholar]
- Rakic S., Bandovic J., Dunjic M., Randjelovic T. Gastric foveolar hyperplasia in patients with cancer of the intact stomach. Surg Laparosc Endosc. 1994 Jun;4(3):196–199. [PubMed] [Google Scholar]
- Robles-Campos R., Lujan-Mompean J. A., Parrilla-Paricio P., Bermejo-Lopez J., Liron-Ruiz R., Torralba-Martinez J. A., Morales-Cuenca G., Molina-Martinzez J. A. Role of Helicobacter pylori infection and duodenogastric reflux in the pathogenesis of alkaline reflux gastritis after gastric operations. Surg Gynecol Obstet. 1993 Jun;176(6):594–598. [PubMed] [Google Scholar]
- Sobala G. M., O'Connor H. J., Dewar E. P., King R. F., Axon A. T., Dixon M. F. Bile reflux and intestinal metaplasia in gastric mucosa. J Clin Pathol. 1993 Mar;46(3):235–240. doi: 10.1136/jcp.46.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. R., Mason R. C., Filipe M. I., Vaja S., Hanley D. C., Murphy G. M., Dowling R. H., McColl I. Gastric carcinogenesis in the rat induced by duodenogastric reflux without carcinogens: morphology, mucin histochemistry, polyamine metabolism, and labelling index. Gut. 1991 Dec;32(12):1447–1454. doi: 10.1136/gut.32.12.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]