Abstract
Background—During medical checkups of two unrelated female outpatients during their annual health examination and one male inpatient suffering from cardiac failure the glycated haemoglobin (HbA1C) concentrations measured by high performance liquid chromatography (HPLC) were low, in spite of normal fasting plasma glucose concentrations. However, HbA1C concentrations measured by latex immunoagglutination and fructosamine concentrations were within the normal range. Method—Investigations were performed to elucidate the reasons for these discrepancies.
Results—Abnormal haemoglobins, Hb Takamatsu and Hb G-Szuhu, were found. The HPLC chromatogram showed an additional peak near HbA1a+b, which resulted in falsely low HbA1C concentrations. Isoelectric focusing analysis of the patients' haemoglobin disclosed abnormal haemoglobins, which migrated faster than normal HbA1 in the two female patients and slower in the male patient. The cDNA sequence and amino acid analyses of the haemoglobin α-chains and ß-chains indicated the presence of the haemoglobin variant ß 120 Lys→Gln in the two female patients and ß 80 Asn→Lys in the male patient; that is, Hb Takamatsu and Hb G-Szuhu.
Conclusions—These cases show how these silent haemoglobin variants can result in falsely low HbA1C concentration readings when using HPLC.
Key Words: abnormal haemoglobin • high performance liquid chromatography • glycated haemoglobin
Full Text
The Full Text of this article is available as a PDF (159.0 KB).
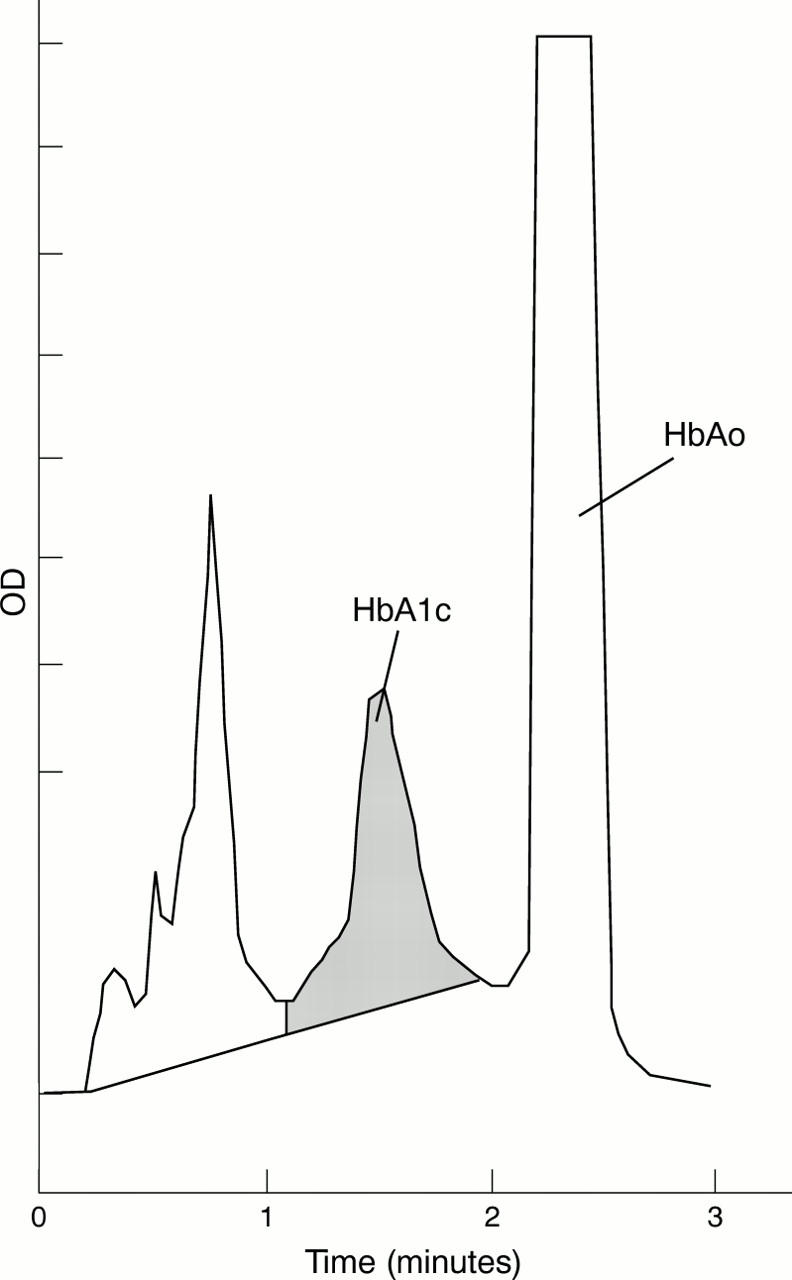
Figure 1 High performance liquid chromatography (HPLC) chromatogram of erythrocyte haemolysates of Hb Takamatsu (patient KM). An additional peak near HbA1a+b caused an underestimation of HbA1C by this method.
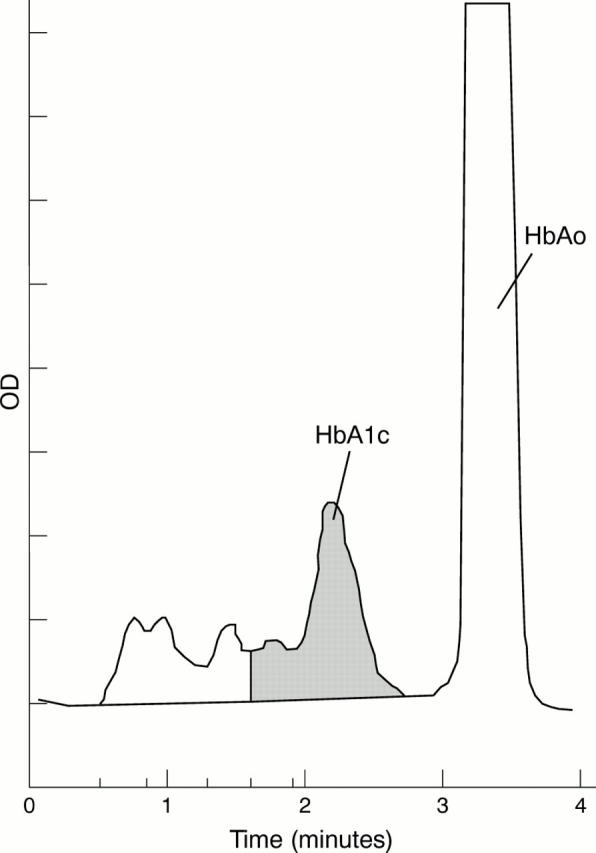
Figure 2 High performance liquid chromatography (HPLC) chromatogram of erythrocyte haemolysates of Hb G-Szuhu (patient ES). An additional peak near HbA1a+b caused an underestimation of HbA1C by this method.
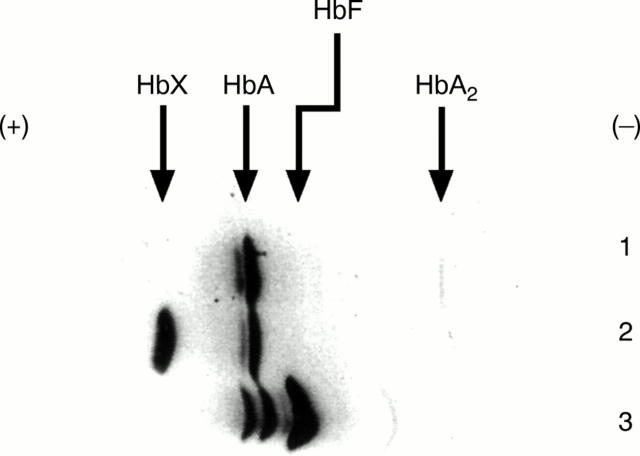
Figure 3 Isoelectric focusing gel electrophoresis of a control subject, cord blood, and the patient with Hb Takamatsu. Lane 1, electrophoretic pattern of control subject; lane 2, electrophoretic pattern of Hb Takamatsu; lane 3, electrophoretic pattern of cord blood. Abnormal haemoglobin migrating faster than HbA1 is indicated by HbX.
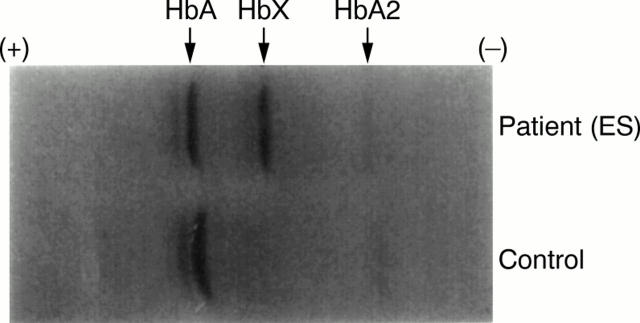
Figure 4 Isoelectric focusing gel electrophoresis of a control subject and the patient with Hb G-Szuhu. Abnormal haemoglobin migrating slower than HbA1 is indicated by HbX.
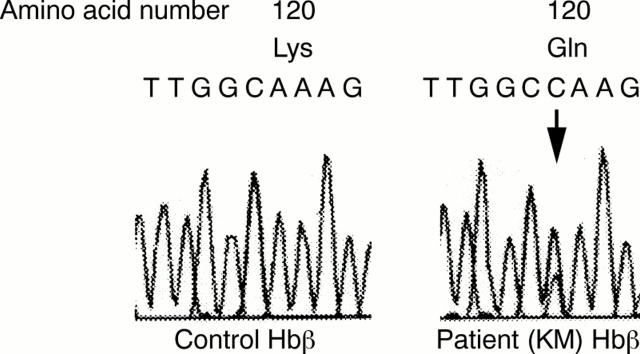
Figure 5 Mutation of amino acid sequence of the Hbß gene at position 120 obtained by means of an ABI PRISM sequencer. The arrow indicates the first nucleotide of the codons for amino acid 120.
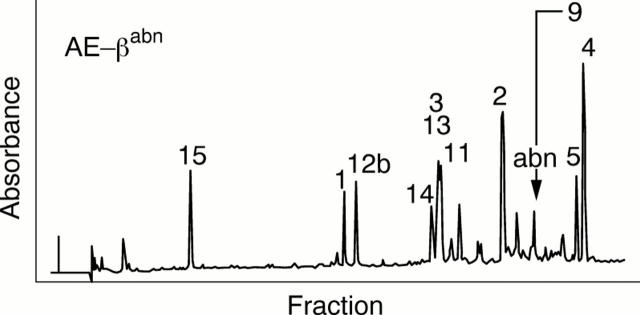
Figure 6 Elution profile of the tryptic digests of aminoethyl abnormal haemoglobin ß of the patient ES (Hb G-Szuhu) by reversed phase high performance liquid chromatography (HPLC).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensinger T. A., Gillette P. N. Hemolysis in sickle cell disease. Arch Intern Med. 1974 Apr;133(4):624–631. [PubMed] [Google Scholar]
- Bisse E., Wieland H. High-performance liquid chromatographic separation of human haemoglobins. Simultaneous quantitation of foetal and glycated haemoglobins. J Chromatogr. 1988 Dec 29;434(1):95–110. doi: 10.1016/0378-4347(88)80065-3. [DOI] [PubMed] [Google Scholar]
- Blackwell R. Q., Yang H. J., Wang C. C. Hemoglobin G Szuhu: beta80 Asn replaced by Lys. Biochim Biophys Acta. 1969 Aug 12;188(1):59–64. doi: 10.1016/0005-2795(69)90045-2. [DOI] [PubMed] [Google Scholar]
- Carver M. F., Huisman T. H. International Hemoglobin Information Center variant list. Hemoglobin. 1996 Aug;20(3):213–213. doi: 10.3109/03630269609027930. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Flückiger R., Harmon W., Meier W., Loo S., Gabbay K. H. Hemoglobin carbamylation in uremia. N Engl J Med. 1981 Apr 2;304(14):823–827. doi: 10.1056/NEJM198104023041406. [DOI] [PubMed] [Google Scholar]
- Hoberman H. D., Chiodo S. M. Elevation of the hemoglobin A1 fraction in alcoholism. Alcohol Clin Exp Res. 1982 Spring;6(2):260–266. doi: 10.1111/j.1530-0277.1982.tb04972.x. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Henson J. B., Wilson J. B. A new high-performance liquid chromatographic procedure to quantitate hemoglobin A1c and other minor hemoglobins in blood of normal, diabetic, and alcoholic individuals. J Lab Clin Med. 1983 Aug;102(2):163–173. [PubMed] [Google Scholar]
- Imai K., Morimoto H., Kotani M., Shibata S., Miyaji T. Studies on the function of abnormal hemoglobins. II. Oxygen equilibrium of abnormal hemoglobins: Shimonoseki, Ube II, Hikari, Gifu, and Agenogi. Biochim Biophys Acta. 1970 Feb 17;200(2):197–202. doi: 10.1016/0005-2795(70)90164-9. [DOI] [PubMed] [Google Scholar]
- Iuchi I., Hidaka K., Harano T., Ueda S., Shibata S., Shimasaki S., Mizushima J., Kubo N., Miyake T., Uchida T. Hemoglobin takamatsu (beta 120 (GH 3) Lys leads to Gln): a new abnormal hemoglobin detected in three unrelated families in the takamatsu area of shikoku. Hemoglobin. 1980;4(2):165–176. doi: 10.3109/03630268009042383. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Yoshimoto K., Hirauchi K., Uchida K. Determination of glycated proteins in biological samples based on colorimetry of 2-keto-glucose released with hydrazine. Biol Pharm Bull. 1994 Mar;17(3):365–369. doi: 10.1248/bpb.17.365. [DOI] [PubMed] [Google Scholar]
- Krishnan K., Martinez F., Wille R. T., Jones R. T., Shih D. T., Head C., Fairbanks V. F., Dabich L. Hb Washtenaw [ beta 11(A8)Val-->Phe]: an electrophorectically silent, unstable, low oxygen affinity variant associated with anemia and chronic cyanosis. Hemoglobin. 1994 Sep;18(4-5):285–295. doi: 10.3109/03630269408996194. [DOI] [PubMed] [Google Scholar]
- Maezawa Y., Yamauchi M., Nakabayashi T., Toshima K., Ikeda K., Toda G. A diabetic case of Hb Riyadh with a low HbA1c value. Intern Med. 1993 Feb;32(2):128–132. doi: 10.2169/internalmedicine.32.128. [DOI] [PubMed] [Google Scholar]
- Nathan D. M., Francis T. B., Palmer J. L. Effect of aspirin on determinations of glycosylated hemoglobin. Clin Chem. 1983 Mar;29(3):466–469. [PubMed] [Google Scholar]
- Ohba Y., Miyaji T., Murakami M., Kadowaki S., Fujita T., Oimomi M., Hatanaka H., Ishikawa K., Baba S., Hitaka K. Hb Himeji or beta 140 (H18) Ala----Asp. A slightly unstable hemoglobin with increased beta N-terminal glycation. Hemoglobin. 1986;10(2):109–125. doi: 10.3109/03630268609046438. [DOI] [PubMed] [Google Scholar]
- Ohba Y. Unstable hemoglobins. Hemoglobin. 1990;14(4):353–388. doi: 10.3109/03630269009031998. [DOI] [PubMed] [Google Scholar]
- Panzer S., Kronik G., Lechner K., Bettelheim P., Neumann E., Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood. 1982 Jun;59(6):1348–1350. [PubMed] [Google Scholar]
- Sandhaus L. M., Harvey F. G. Laboratory methods for the detection of hemoglobinopathies in the community hospital. Clin Lab Med. 1993 Dec;13(4):801–816. [PubMed] [Google Scholar]
- Schnedl W. J., Reisinger E. C., Katzensteiner S., Lipp R. W., Schreiber F., Hopmeier P., Krejs G. J. Haemoglobin O Padova and falsely low haemoglobin A1c in a patient with type I diabetes. J Clin Pathol. 1997 May;50(5):434–435. doi: 10.1136/jcp.50.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnedl W. J., Reisinger E. C., Pieber T. R., Lipp R. W., Schreiber F., Hopmeier P., Krejs G. J. Hemoglobin Sherwood Forest detected by high performance liquid chromatography for hemoglobin A1c. Am J Clin Pathol. 1995 Oct;104(4):444–446. doi: 10.1093/ajcp/104.4.444. [DOI] [PubMed] [Google Scholar]
- Shinar E., Rachmilewitz E. A. Differences in the pathophysiology of hemolysis of alpha- and beta-thalassemic red blood cells. Ann N Y Acad Sci. 1990;612:118–126. doi: 10.1111/j.1749-6632.1990.tb24297.x. [DOI] [PubMed] [Google Scholar]
- Shiwa M., Fukuda K., Kawata Y., Matsuura T., Nagao S., Matsuura T., Katsuta I., Hirano M., Ohba Y., Miyaji T. [Abnormal hemoglobin (Hb Camden) detected by interference in the determination of glycosylated hemoglobin with high performance liquid chromatography]. Rinsho Byori. 1988 Jul;36(7):866–870. [PubMed] [Google Scholar]
- Stabler S. P., Jones R. T., Head C., Shih D. T., Fairbanks V. F. Hemoglobin Denver [alpha 2 beta 2(41) (C7) Phe-->Ser]: a low-O2-affinity variant associated with chronic cyanosis and anemia. Mayo Clin Proc. 1994 Mar;69(3):237–243. doi: 10.1016/s0025-6196(12)61062-3. [DOI] [PubMed] [Google Scholar]
- Trivelli L. A., Ranney H. M., Lai H. T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971 Feb 18;284(7):353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- Wajcman H., Galactéros F. Abnormal hemoglobins with high oxygen affinity and erythrocytosis. Hematol Cell Ther. 1996 Aug;38(4):305–312. doi: 10.1007/s00282-996-0305-4. [DOI] [PubMed] [Google Scholar]
- Wong S. C., Tesanovic M., Poon M. C. Detection of two abnormal hemoglobins, Hb Manitoba and Hb G-Coushatta, during analysis of glycohemoglobin (A1c) by high-performance liquid chromatography. Clin Chem. 1991 Aug;37(8):1456–1459. [PubMed] [Google Scholar]
- Yagame M., Jinde K., Suzuki D., Saotome N., Takano H., Tanabe R., Sato H., Kurokawa K., Sakai H., Matsumae M. A diabetic case with hemoglobin J-Meerut and low HbA1C levels. Intern Med. 1997 May;36(5):351–356. doi: 10.2169/internalmedicine.36.351. [DOI] [PubMed] [Google Scholar]