Abstract
Aims—To assess A103 (melan-A) immunoreactivity in a range of ovarian sex cord stromal tumours and to evaluate it for the differential diagnosis of other neoplasms.
Methods—Paraffin embedded tissue sections from 45 sex cord stromal tumours and 44 potential histological mimics were examined immunohistochemically using the antibody A103. The sex cord stromal group included 21 adult granulosa cell tumours (AGCT), two juvenile granulosa cell tumours (JGCT), eight tumours showing Sertoli cell or Sertoli-Leydig cell differentiation, two unclassified tumours, two gonadoblastomas, one sex cord tumour with annular tubules, two steroid cell tumours, five thecomas/fibrothecomas, and two sclerosing stromal tumours. The histological mimics include 14 primary ovarian carcinomas, 13 metastatic carcinomas, four carcinoid tumours, four lymphomas, three endometrioid stromal sarcomas, two ovarian tumours of probable Wolffian origin, and one case each of small cell carcinoma, desmoplastic small round cell tumour, melanoma, and primitive neuroectodermal tumour.
Results—A103 immunoreactivity was identified in 25 sex cord stromal tumours including 10 AGCT, two JGCT, six Sertoli/Sertoli-Leydig cell tumours, two steroid cell tumours, three thecomas/fibrothecomas, and two sclerosing stromal tumours. Of the potential histological mimics, staining was present only in the two ovarian tumours of probable Wolffian origin and the melanoma. Immunoreactive stromal cells were noted in a minority of cases. Normal hilus cells and rete ovarii epithelium also expressed A103.
Conclusions—A103 is a moderately sensitive and specific marker of sex cord stromal differentiation within the range of tumours examined in this study and as such is a valuable adjunct to other immunocytochemical markers in the assessment of diagnostically problematic ovarian tumours. The staining of normal and neoplastic Wolffian elements merits further investigation.
Key Words: ovarian tumours • A103 • immunohistochemistry
Full Text
The Full Text of this article is available as a PDF (235.8 KB).
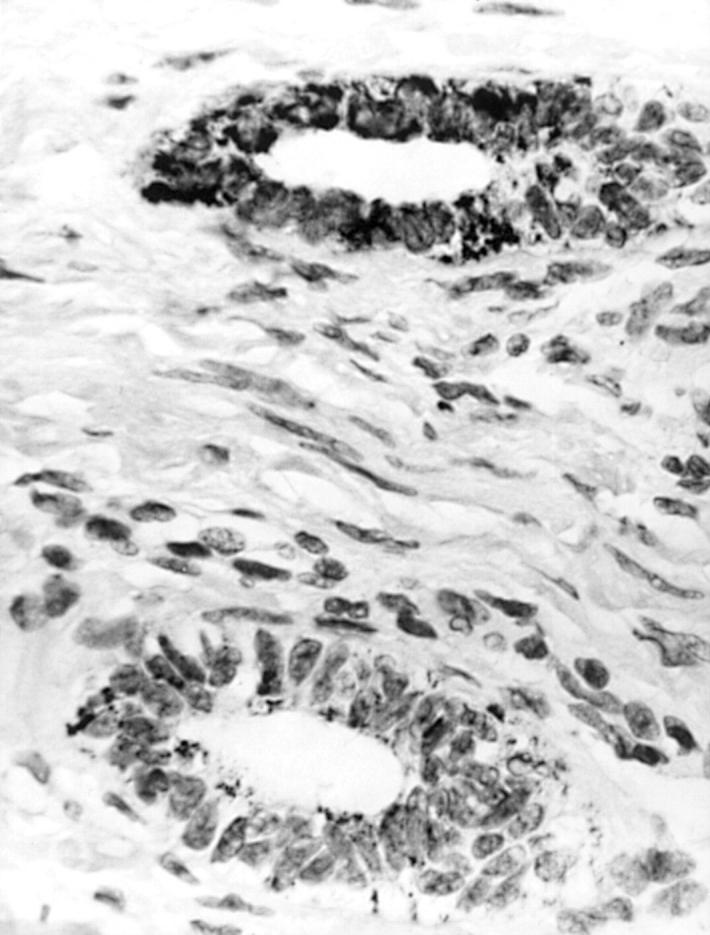
Figure 1 A103 immunoreactivity is focally present in tubules of the normal rete ovarii.
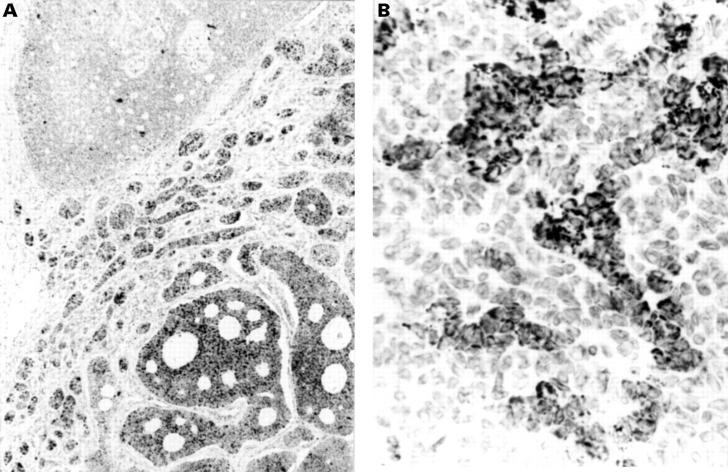
Figure 2 Adult granulosa cell tumour shows strong but focal A103 expression within (A) microfollicular (lower right) and (B) trabecular areas.
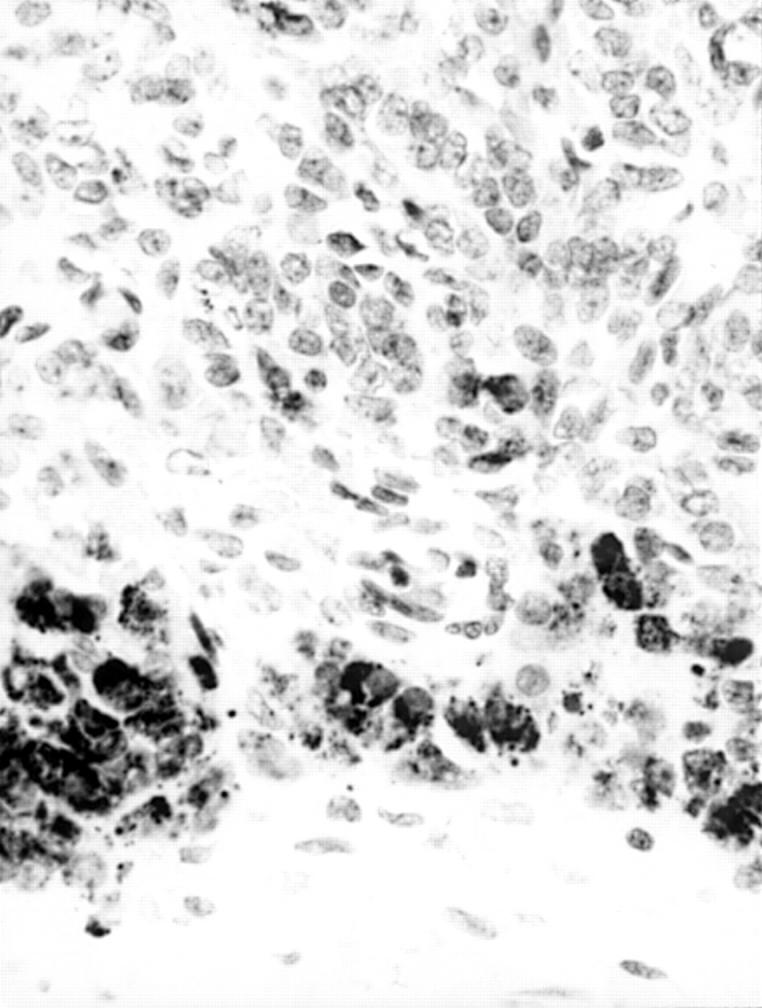
Figure 3 Peripheral Leydig cell clusters (below) are strongly A103 immunoreactive in a poorly differentiated Sertoli-Leydig cell tumour. Sertoli cell elements (above) are mainly unstained.

Figure 4 Steroid cell tumour (above) shows diffuse, strong A103 expression. Immunoreactive hilus cells are also present adjacent to medullary nerves (arrow).
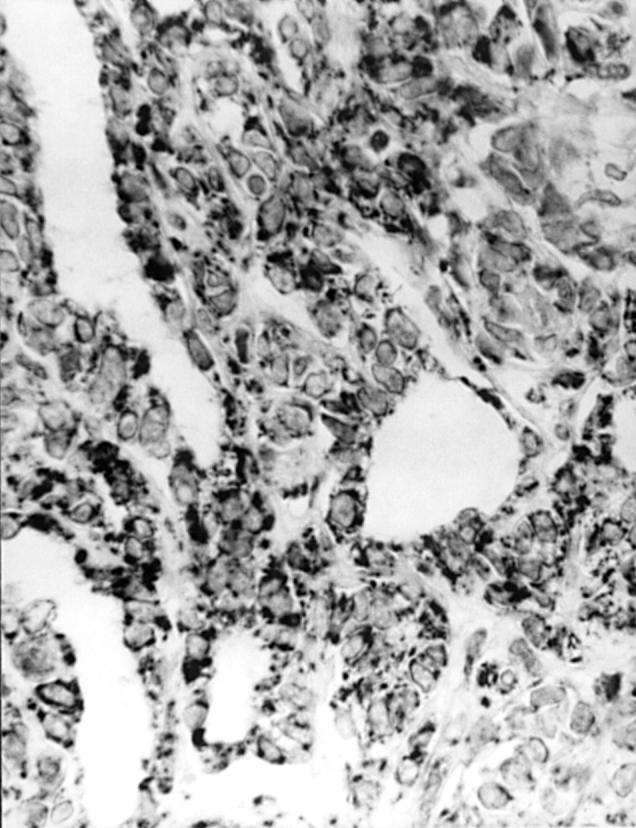
Figure 5 Ovarian tumour of probable Wolffian origin shows strong A103 immunoreactivity. Other areas of tumour were only focally stained.
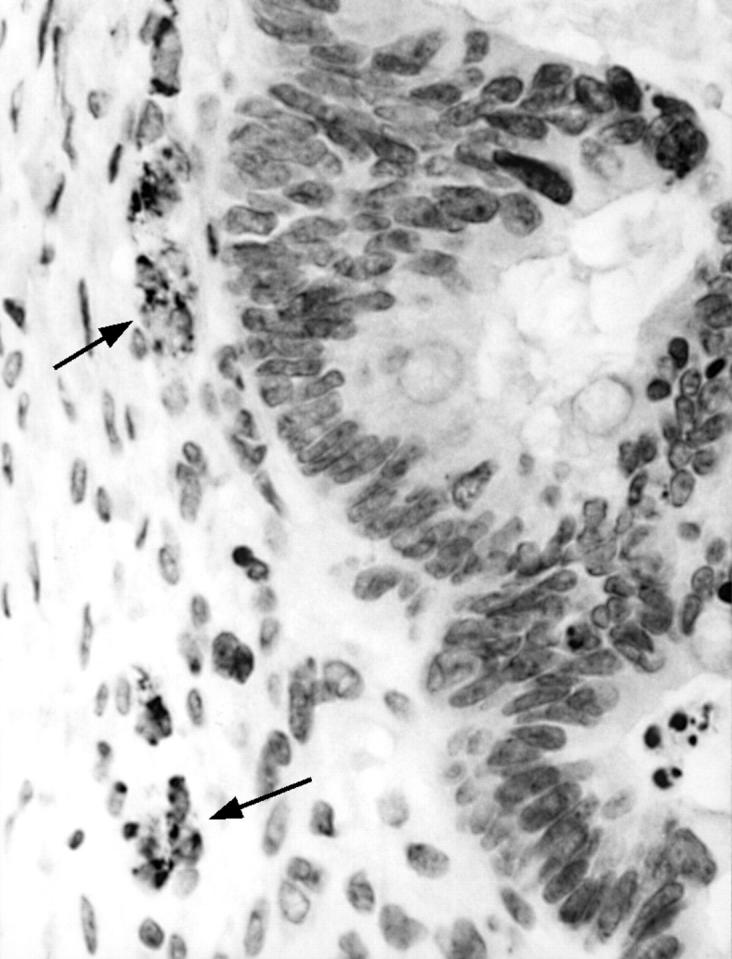
Figure 6 Focal A103 expression is seen in reactive stromal cells (arrows) but endometrioid carcinoma (right) is not stained.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blessing K., Sanders D. S., Grant J. J. Comparison of immunohistochemical staining of the novel antibody melan-A with S100 protein and HMB-45 in malignant melanoma and melanoma variants. Histopathology. 1998 Feb;32(2):139–146. doi: 10.1046/j.1365-2559.1998.00312.x. [DOI] [PubMed] [Google Scholar]
- Busam K. J., Chen Y. T., Old L. J., Stockert E., Iversen K., Coplan K. A., Rosai J., Barnhill R. L., Jungbluth A. A. Expression of melan-A (MART1) in benign melanocytic nevi and primary cutaneous malignant melanoma. Am J Surg Pathol. 1998 Aug;22(8):976–982. doi: 10.1097/00000478-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Busam K. J., Iversen K., Coplan K. A., Old L. J., Stockert E., Chen Y. T., McGregor D., Jungbluth A. Immunoreactivity for A103, an antibody to melan-A (Mart-1), in adrenocortical and other steroid tumors. Am J Surg Pathol. 1998 Jan;22(1):57–63. doi: 10.1097/00000478-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Chen Y. T., Stockert E., Jungbluth A., Tsang S., Coplan K. A., Scanlan M. J., Old L. J. Serological analysis of Melan-A(MART-1), a melanocyte-specific protein homogeneously expressed in human melanomas. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5915–5919. doi: 10.1073/pnas.93.12.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. J., DeRose P. B., Roth L. M., Brescia R. J., Zaloudek C. J., Cohen C. Immunohistochemical phenotype of ovarian granulosa cell tumors: absence of epithelial membrane antigen has diagnostic value. Hum Pathol. 1994 Jan;25(1):60–66. doi: 10.1016/0046-8177(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Coulie P. G., Brichard V., Van Pel A., Wölfel T., Schneider J., Traversari C., Mattei S., De Plaen E., Lurquin C., Szikora J. P. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994 Jul 1;180(1):35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming P., Wellmann A., Maschek H., Lang H., Georgii A. Monoclonal antibodies against inhibin represent key markers of adult granulosa cell tumors of the ovary even in their metastases. A report of three cases with late metastasis, being previously misinterpreted as hemangiopericytoma. Am J Surg Pathol. 1995 Aug;19(8):927–933. doi: 10.1097/00000478-199508000-00008. [DOI] [PubMed] [Google Scholar]
- Fox H. Sex cord-stromal tumours of the ovary. J Pathol. 1985 Feb;145(2):127–148. doi: 10.1002/path.1711450202. [DOI] [PubMed] [Google Scholar]
- Gordon M. D., Corless C., Renshaw A. A., Beckstead J. CD99, keratin, and vimentin staining of sex cord-stromal tumors, normal ovary, and testis. Mod Pathol. 1998 Aug;11(8):769–773. [PubMed] [Google Scholar]
- Hildebrandt R. H., Rouse R. V., Longacre T. A. Value of inhibin in the identification of granulosa cell tumors of the ovary. Hum Pathol. 1997 Dec;28(12):1387–1395. doi: 10.1016/s0046-8177(97)90229-x. [DOI] [PubMed] [Google Scholar]
- Jungbluth A. A., Busam K. J., Gerald W. L., Stockert E., Coplan K. A., Iversen K., MacGregor D. P., Old L. J., Chen Y. T. A103: An anti-melan-a monoclonal antibody for the detection of malignant melanoma in paraffin-embedded tissues. Am J Surg Pathol. 1998 May;22(5):595–602. doi: 10.1097/00000478-199805000-00011. [DOI] [PubMed] [Google Scholar]
- Kaufmann O., Koch S., Burghardt J., Audring H., Dietel M. Tyrosinase, melan-A, and KBA62 as markers for the immunohistochemical identification of metastatic amelanotic melanomas on paraffin sections. Mod Pathol. 1998 Aug;11(8):740–746. [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Rivoltini L., Topalian S. L., Miki T., Rosenberg S. A. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommoss F., Oliva E., Bhan A. K., Young R. H., Scully R. E. Inhibin expression in ovarian tumors and tumor-like lesions: an immunohistochemical study. Mod Pathol. 1998 Jul;11(7):656–664. [PubMed] [Google Scholar]
- Koonings P. P., Campbell K., Mishell D. R., Jr, Grimes D. A. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989 Dec;74(6):921–926. [PubMed] [Google Scholar]
- Krishnamurthy S., Jungbluth A. A., Busam K. J., Rosai J. Uterine tumors resembling ovarian sex-cord tumors have an immunophenotype consistent with true sex-cord differentiation. Am J Surg Pathol. 1998 Sep;22(9):1078–1082. doi: 10.1097/00000478-199809000-00006. [DOI] [PubMed] [Google Scholar]
- Loo K. T., Leung A. K., Chan J. K. Immunohistochemical staining of ovarian granulosa cell tumours with MIC2 antibody. Histopathology. 1995 Oct;27(4):388–390. doi: 10.1111/j.1365-2559.1995.tb01534.x. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Maxwell P., Sloan J. M. Immunohistochemical staining of ovarian granulosa cell tumors with monoclonal antibody against inhibin. Hum Pathol. 1997 Sep;28(9):1034–1038. doi: 10.1016/s0046-8177(97)90056-3. [DOI] [PubMed] [Google Scholar]
- Pelkey T. J., Frierson H. F., Jr, Mills S. E., Stoler M. H. The diagnostic utility of inhibin staining in ovarian neoplasms. Int J Gynecol Pathol. 1998 Apr;17(2):97–105. doi: 10.1097/00004347-199804000-00001. [DOI] [PubMed] [Google Scholar]
- Rahilly M. A., Williams A. R., Krausz T., al Nafussi A. Female adnexal tumour of probable Wolffian origin: a clinicopathological and immunohistochemical study of three cases. Histopathology. 1995 Jan;26(1):69–74. doi: 10.1111/j.1365-2559.1995.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Rishi M., Howard L. N., Bratthauer G. L., Tavassoli F. A. Use of monoclonal antibody against human inhibin as a marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol. 1997 May;21(5):583–589. doi: 10.1097/00000478-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Roth L. M., Liban E., Czernobilsky B. Ovarian endometrioid tumors mimicking Sertoli and Sertoli-Leydig cell tumors: Sertoliform variant of endometrioid carcinoma. Cancer. 1982 Oct 1;50(7):1322–1331. doi: 10.1002/1097-0142(19821001)50:7<1322::aid-cncr2820500718>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Scully R. E. Gonadoblastoma. A review of 74 cases. Cancer. 1970 Jun;25(6):1340–1356. doi: 10.1002/1097-0142(197006)25:6<1340::aid-cncr2820250612>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Seidman J. D. Unclassified ovarian gonadal stromal tumors. A clinicopathologic study of 32 cases. Am J Surg Pathol. 1996 Jun;20(6):699–706. doi: 10.1097/00000478-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Stewart C. J., Jeffers M. D., Kennedy A. Diagnostic value of inhibin immunoreactivity in ovarian gonadal stromal tumours and their histological mimics. Histopathology. 1997 Jul;31(1):67–74. doi: 10.1046/j.1365-2559.1997.5780819.x. [DOI] [PubMed] [Google Scholar]
- Tornos C., Silva E. G., Ordonez N. G., Gershenson D. M., Young R. H., Scully R. E. Endometrioid carcinoma of the ovary with a prominent spindle-cell component, a source of diagnostic confusion. A report of 14 cases. Am J Surg Pathol. 1995 Dec;19(12):1343–1353. doi: 10.1097/00000478-199512000-00001. [DOI] [PubMed] [Google Scholar]
- Young R. H. Ovarian tumors other than those of surface epithelial-stromal type. Hum Pathol. 1991 Aug;22(8):763–775. doi: 10.1016/0046-8177(91)90206-5. [DOI] [PubMed] [Google Scholar]
- Young R. H., Scully R. E. Malignant melanoma metastatic to the ovary. A clinicopathologic analysis of 20 cases. Am J Surg Pathol. 1991 Sep;15(9):849–860. doi: 10.1097/00000478-199109000-00005. [DOI] [PubMed] [Google Scholar]
- Young R. H., Scully R. E. Ovarian sex cord-stromal tumors. Problems in differential diagnosis. Pathol Annu. 1988;23(Pt 1):237–296. [PubMed] [Google Scholar]
- Young R. H., Welch W. R., Dickersin G. R., Scully R. E. Ovarian sex cord tumor with annular tubules: review of 74 cases including 27 with Peutz-Jeghers syndrome and four with adenoma malignum of the cervix. Cancer. 1982 Oct 1;50(7):1384–1402. doi: 10.1002/1097-0142(19821001)50:7<1384::aid-cncr2820500726>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]