Abstract
Background—A transient expansion of the CD8+ T cell pool normally occurs in the early phase of HIV infection. Persistent expansion of this pool is observed in two related settings: diffuse infiltrative lymphocytosis syndrome (DILS) and HIV associated CD8+ lymphocytosis syndrome.
Aim—To investigate a group of HIV infected patients with CD8+ lymphocytosis syndrome with particular emphasis on whether monoclonality was present.
Methods—A group of 18 patients with HIV-1 infection and persistent circulating CD8+ lymphocytosis was compared with 21 HIV positive controls. Serum samples were tested for antinuclear antibodies, antibodies to extractable nuclear antigens, immunoglobulin levels, paraproteins, human T lymphotropic virus type 1 (HTLV-1), Epstein-Barr virus, and cytomegalovirus serology. Lymphocyte phenotyping and HLA-DR typing was performed, and T cell receptor (TCR) gene rearrangement studies used to identify monoclonal populations of T cells. CD4+ and CD8+ subsets of peripheral blood lymphocytes were purified to determine whether CD8+ populations inhibited HIV replication in autologous CD4+ cells.
Results—A subgroup of patients with HIV-1 infection was found to have expanded populations of CD8+ T cell large granular lymphocytes persisting for 6 to 30 months. The consensus immunophenotype was CD4- CD8+ DRhigh CD11a+ CD11c+ CD16- CD28± CD56- CD57+, consistent with typical T cell large granular lymphocytes expressing cellular activation markers. Despite the finding of monoclonal TCR gene usage in five of 18 patients, there is evidence that the CD8+ expansions are reactive populations capable of mediating non-cytotoxic inhibition of HIV replication.
Conclusions—A subgroup of HIV positive patients has CD8+ lymphocytosis, but despite the frequent occurrence of monoclonal TCR gene usage there is evidence that this represents an immune response to viral infection rather than a malignant disorder.
Key Words: HIV infection • CD8+ lymphocytosis • clonality
Full Text
The Full Text of this article is available as a PDF (135.4 KB).
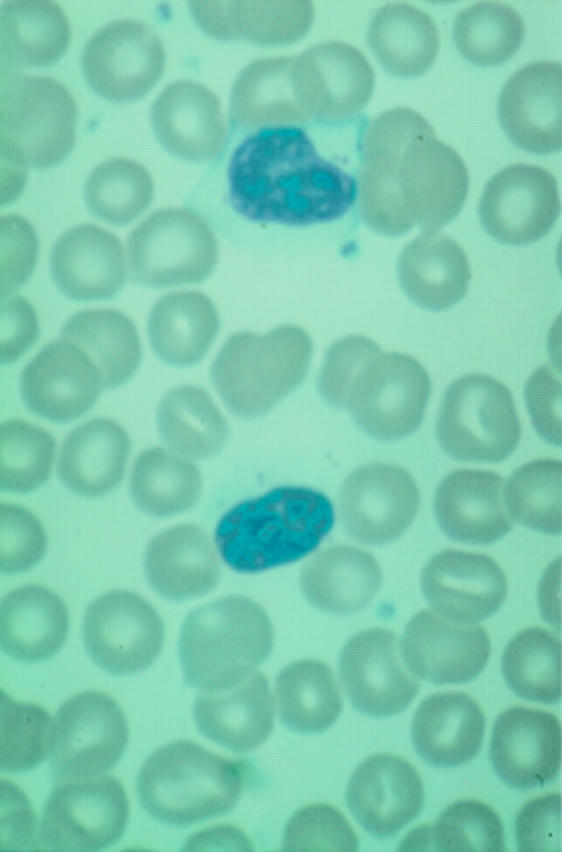
Figure 1 Peripheral blood film showing T cell large granular lymphocytes in a patient with CD8+ lymphocytosis (x270).
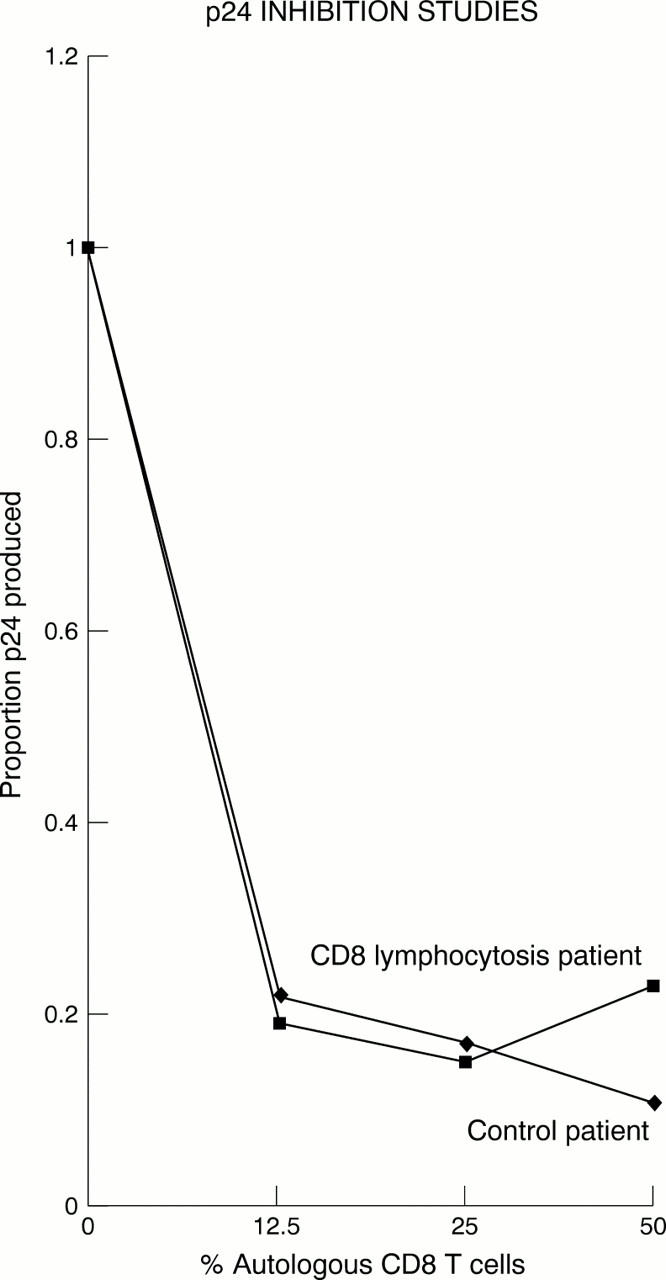
Figure 2 Proportion of p24 produced against percentage of CD8+ T cells. As autologous CD8+ T cells are reintroduced to CD4+ T cells in increasing concentrations, p24 production is inhibited. (Results from a single representative experiment.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Geoffroy C., Klatzmann D., Gluckman J. C., Gruest J., Montagnier L. High production of the acquired immunodeficiency syndrome virus (lymphadenopathy-associated virus) by human T lymphocytes stimulated by streptococcal mitogenic toxins. J Clin Microbiol. 1986 Oct;24(4):639–641. doi: 10.1128/jcm.24.4.639-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinchmann J. E., Gaudernack G., Vartdal F. CD8+ T cells inhibit HIV replication in naturally infected CD4+ T cells. Evidence for a soluble inhibitor. J Immunol. 1990 Apr 15;144(8):2961–2966. [PubMed] [Google Scholar]
- Brugnoni D., Prati E., Malacarne F., Gorla R., Airò P., Cattaneo R. The primary response to HIV infection is characterized by an expansion of activated CD8+ CD28- cells. AIDS. 1996 Jan;10(1):104–106. doi: 10.1097/00002030-199601000-00017. [DOI] [PubMed] [Google Scholar]
- Constans J., Ladner J., Dabis F., Brossard G., Commenges D., Leng B., Conri C. Hyperlymphocytose CD8 au cours de l'infection par le VIH. 63 observations. Presse Med. 1992 Jan 4;21(1):27–30. [PubMed] [Google Scholar]
- Dwyer E., Itescu S., Winchester R. Characterization of the primary structure of T cell receptor beta chains in cells infiltrating the salivary gland in the sicca syndrome of HIV-1 infection. Evidence of antigen-driven clonal selection suggested by restricted combinations of V beta J beta gene segment usage and shared somatically encoded amino acid residues. J Clin Invest. 1993 Jul;92(1):495–502. doi: 10.1172/JCI116593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. M., Lawrence J., Schapiro J. M., Altman J. D., Winters M. A., Crompton M., Loi M., Kundu S. K., Davis M. M., Merigan T. C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART). J Immunol. 1999 Feb 1;162(3):1780–1788. [PubMed] [Google Scholar]
- Halapi E., Gigliotti D., Hodara V., Scarlatti G., Tovo P. A., DeMaria A., Wigzell H., Rossi P. Detection of CD8 T-cell expansions with restricted T-cell receptor V gene usage in infants vertically infected by HIV-1. AIDS. 1996 Dec;10(14):1621–1626. doi: 10.1097/00002030-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Itescu S., Brancato L. J., Buxbaum J., Gregersen P. K., Rizk C. C., Croxson T. S., Solomon G. E., Winchester R. A diffuse infiltrative CD8 lymphocytosis syndrome in human immunodeficiency virus (HIV) infection: a host immune response associated with HLA-DR5. Ann Intern Med. 1990 Jan 1;112(1):3–10. doi: 10.7326/0003-4819-112-1-3. [DOI] [PubMed] [Google Scholar]
- Itescu S., Dalton J., Zhang H. Z., Winchester R. Tissue infiltration in a CD8 lymphocytosis syndrome associated with human immunodeficiency virus-1 infection has the phenotypic appearance of an antigenically driven response. J Clin Invest. 1993 May;91(5):2216–2225. doi: 10.1172/JCI116448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itescu S., Rose S., Dwyer E., Winchester R. Certain HLA-DR5 and -DR6 major histocompatibility complex class II alleles are associated with a CD8 lymphocytic host response to human immunodeficiency virus type 1 characterized by low lymphocyte viral strain heterogeneity and slow disease progression. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11472–11476. doi: 10.1073/pnas.91.24.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi S., Cohen P. R., Williams F., Schempp R., Reveille J. D. The diffuse infiltrative lymphocytosis syndrome. Clinical and immunogenetic features in 35 patients. AIDS. 1996 Apr;10(4):385–391. [PubMed] [Google Scholar]
- Livingstone W. J., Moore M., Innes D., Bell J. E., Simmonds P. Frequent infection of peripheral blood CD8-positive T-lymphocytes with HIV-1. Edinburgh Heterosexual Transmission Study Group. Lancet. 1996 Sep 7;348(9028):649–654. doi: 10.1016/s0140-6736(96)02091-0. [DOI] [PubMed] [Google Scholar]
- Lynas C., Howe D. Simple, reliable detection of T cell clones by PCR-LIS-SSCP analysis of TCRgamma rearrangement. Mol Cell Probes. 1998 Feb;12(1):41–48. doi: 10.1006/mcpr.1997.0146. [DOI] [PubMed] [Google Scholar]
- Moulignier A., Authier F. J., Baudrimont M., Pialoux G., Belec L., Polivka M., Clair B., Gray F., Mikol J., Gherardi R. K. Peripheral neuropathy in human immunodeficiency virus-infected patients with the diffuse infiltrative lymphocytosis syndrome. Ann Neurol. 1997 Apr;41(4):438–445. doi: 10.1002/ana.410410406. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J. D., Suresh M., Sourdive D. J., Zajac A. J., Miller J. D., Slansky J., Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998 Feb;8(2):177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Nakahara K., Utsunomiya A., Hanada S., Takeshita T., Uozumi K., Yamamoto K., Komatsu H., Nitta M., Ueda R., Tatsumi E. Transient appearance of CD3+CD8+ T lymphocytes with monoclonal gene rearrangement of T-cell receptor beta locus. Br J Haematol. 1998 Feb;100(2):411–414. doi: 10.1046/j.1365-2141.1998.00555.x. [DOI] [PubMed] [Google Scholar]
- Pollack H., Zhan M. X., Safrit J. T., Chen S. H., Rochford G., Tao P. Z., Koup R., Krasinski K., Borkowsky W. CD8+ T-cell-mediated suppression of HIV replication in the first year of life: association with lower viral load and favorable early survival. AIDS. 1997 Jan;11(1):F9–13. doi: 10.1097/00002030-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Pulik M., Lionnet F., Genet P., Petitdidier C., Jary L., Fourcade C. CD3+ CD8+ CD56- clonal large granular lymphocyte leukaemia and HIV infection. Br J Haematol. 1997 Aug;98(2):444–445. doi: 10.1046/j.1365-2141.1997.1913009.x. [DOI] [PubMed] [Google Scholar]
- Roos M. T., Lange J. M., de Goede R. E., Coutinho R. A., Schellekens P. T., Miedema F., Tersmette M. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J Infect Dis. 1992 Mar;165(3):427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- Schwab R., Szabo P., Manavalan J. S., Weksler M. E., Posnett D. N., Pannetier C., Kourilsky P., Even J. Expanded CD4+ and CD8+ T cell clones in elderly humans. J Immunol. 1997 May 1;158(9):4493–4499. [PubMed] [Google Scholar]
- Strickler J. G., Movahed L. A., Gajl-Peczalska K. J., Horwitz C. A., Brunning R. D., Weiss L. M. Oligoclonal T cell receptor gene rearrangements in blood lymphocytes of patients with acute Epstein-Barr virus-induced infectious mononucleosis. J Clin Invest. 1990 Oct;86(4):1358–1363. doi: 10.1172/JCI114847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Levy J. A. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology. 1989 Apr;66(4):628–630. [PMC free article] [PubMed] [Google Scholar]
- Zambello R., Trentin L., Agostini C., Francia di Celle P., Francavilla E., Barelli A., Cerutti A., Siviero F., Foà R., Semenzato G. Persistent polyclonal lymphocytosis in human immunodeficiency virus-1-infected patients. Blood. 1993 Jun 1;81(11):3015–3021. [PubMed] [Google Scholar]


