Abstract
Background—Increased dietary calcium might reduce colorectal cancer risk, possibly by reduction of colonic epithelial hyperproliferation, but not all studies have demonstrated this. Little is known about the effects of calcium on colonic apoptosis.
Aim—To quantify the effects of increasing calcium on apoptosis and cell proliferation in normal murine colonic crypt epithelium.
Methods—Twenty one day old male C57Bl/6 mice were fed either control AIN-76 diet (0.5% calcium wt/wt; n = 10) or the same supplemented with calcium carbonate (1.0% calcium; n = 10) for 12 weeks. Apoptotic cells in proximal and distal segments were counted and expressed as an apoptotic index (AI: frequency of apoptosis/100 longitudinal crypts). The bromodeoxyuridine (BrdU) labelling index was also determined. Differences were analysed by the student's t test.
Results—In control animals, the AI was significantly higher in the caecum/proximal colon (mean, 28.6; SEM, 2.0) compared with the distal colon (mean, 19.9; SEM, 1.8; p = 0.004). In the calcium treated group, the AI in the caecum/proximal colon (mean, 30.6; SEM, 1.7) was similar to controls (p = 0.71) but the AI in the distal colon was significantly greater (mean, 32.6; SEM, 1.8; p = 0.001) than in control mice and was raised to values similar to those in the proximal colon. Calcium was also associated with reduced crypt cellularity and, in the proximal colon, a downward shift in the crypt position at which apoptosis occurred. There were no significant differences in the BrdU labelling index between groups or between proximal and distal colonic segments in each group.
Conclusions—Increased dietary calcium is associated with the induction of apoptosis in normal mouse distal colonic epithelium without affecting cell proliferation. This might contribute to its putative chemopreventive role in colorectal carcinogenesis. Whether this effect is direct or indirect requires further study.
Key Words: calcium • apoptosis • colonic neoplasms
Full Text
The Full Text of this article is available as a PDF (147.6 KB).

Figure 1 (A) Photomicrograph showing an apoptotic cell in the proliferative compartment near the base of a crypt in the distal colon of a calcium treated mouse (arrow). The apoptotic cell appears within a vacuole and contains several spheres of condensed chromatin. Haematoxylin and eosin stained, x630 magnification. (B) Photomicrograph of four bromodeoxyuridine labelled cells in the proliferative compartment of a crypt in the distal colon of a calcium treated mouse. Immunoperoxidase staining, x630 magnification.
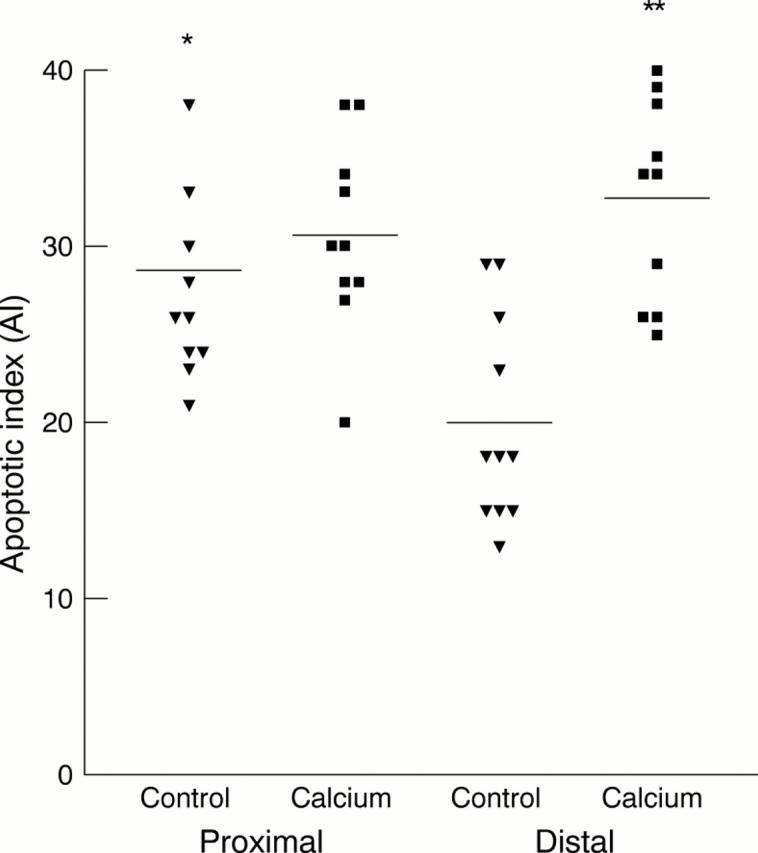
Figure 2 Apoptotic index (expressed as total number of apoptoses for each 100 intact crypts) in proximal and distal colonic segments of control (black triangles) and calcium treated (black squares) mice. *p = 0.01 compared with control distal colon. **p = 0.001 compared with control distal colon.
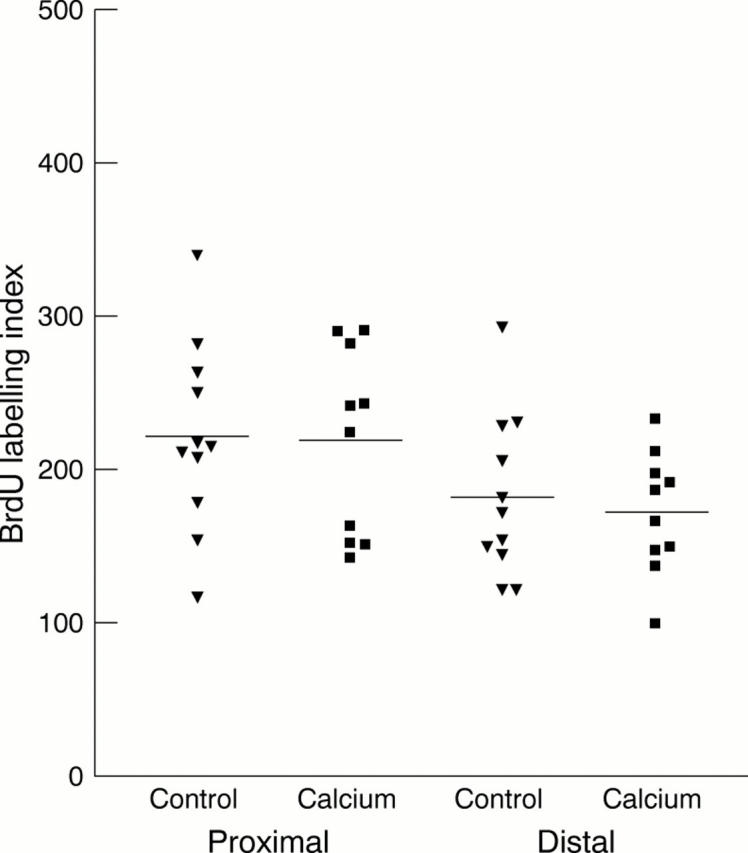
Figure 3 Bromodeoxyuridine (BrdU) labelling index (total number of labelled cells in each 100 intact crypts) in proximal and distal colonic segments of control (black triangles) and calcium treated (black squares) mice. No significant differences were seen between groups or between the proximal and distal colon in either group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton G. V., Davies P. W., Bristol J. B., Williamson R. C. Inhibition of intestinal carcinogenesis by dietary supplementation with calcium. Br J Surg. 1987 Jun;74(6):523–525. doi: 10.1002/bjs.1800740635. [DOI] [PubMed] [Google Scholar]
- Arbman G., Axelson O., Ericsson-Begodzki A. B., Fredriksson M., Nilsson E., Sjödahl R. Cereal fiber, calcium, and colorectal cancer. Cancer. 1992 Apr 15;69(8):2042–2048. doi: 10.1002/1097-0142(19920415)69:8<2042::aid-cncr2820690806>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Armitage N. C., Rooney P. S., Gifford K. A., Clarke P. A., Hardcastle J. D. The effect of calcium supplements on rectal mucosal proliferation. Br J Cancer. 1995 Jan;71(1):186–190. doi: 10.1038/bjc.1995.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J. A., Beach M., Mandel J. S., van Stolk R. U., Haile R. W., Sandler R. S., Rothstein R., Summers R. W., Snover D. C., Beck G. J. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999 Jan 14;340(2):101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- Barsoum G. H., Thompson H., Neoptolemos J. P., Keighley M. R. Dietary calcium does not reduce experimental colorectal carcinogenesis after small bowel resection despite reducing cellular proliferation. Gut. 1992 Nov;33(11):1515–1520. doi: 10.1136/gut.33.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty M. M., Lee E. Y., Glauert H. P. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J Nutr. 1993 Jan;123(1):144–152. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- Bedi A., Pasricha P. J., Akhtar A. J., Barber J. P., Bedi G. C., Giardiello F. M., Zehnbauer B. A., Hamilton S. R., Jones R. J. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995 May 1;55(9):1811–1816. [PubMed] [Google Scholar]
- Bostick R. M., Potter J. D., Fosdick L., Grambsch P., Lampe J. W., Wood J. R., Louis T. A., Ganz R., Grandits G. Calcium and colorectal epithelial cell proliferation: a preliminary randomized, double-blinded, placebo-controlled clinical trial. J Natl Cancer Inst. 1993 Jan 20;85(2):132–141. doi: 10.1093/jnci/85.2.132. [DOI] [PubMed] [Google Scholar]
- Buset M., Lipkin M., Winawer S., Swaroop S., Friedman E. Inhibition of human colonic epithelial cell proliferation in vivo and in vitro by calcium. Cancer Res. 1986 Oct;46(10):5426–5430. [PubMed] [Google Scholar]
- Cross H. S., Pavelka M., Slavik J., Peterlik M. Growth control of human colon cancer cells by vitamin D and calcium in vitro. J Natl Cancer Inst. 1992 Sep 2;84(17):1355–1357. doi: 10.1093/jnci/84.17.1355. [DOI] [PubMed] [Google Scholar]
- Garland C., Shekelle R. B., Barrett-Connor E., Criqui M. H., Rossof A. H., Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985 Feb 9;1(8424):307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- Hall C., Youngs D., Keighley M. R. Crypt cell production rates at various sites around the colon in Wistar rats and humans. Gut. 1992 Nov;33(11):1528–1531. doi: 10.1136/gut.33.11.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby R. F., Bolt M. J., Dolan M. E., Otto G., Dudeja P., Sitrin M. D., Brasitus T. A. Supplemental dietary calcium fails to alter the acute effects of 1,2-dimethylhydrazine on O6-methylguanine, O6-alkylguanine-DNA alkyltransferase and cellular proliferation in the rat colon. Carcinogenesis. 1993 Jun;14(6):1175–1179. doi: 10.1093/carcin/14.6.1175. [DOI] [PubMed] [Google Scholar]
- Karkare M. R., Clark T. D., Glauert H. P. Effect of dietary calcium on colon carcinogenesis induced by a single injection of 1,2-dimethylhydrazine in rats. J Nutr. 1991 Apr;121(4):568–577. doi: 10.1093/jn/121.4.568. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Canman C. E., Leonard C. J. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995 Mar;14(1):3–15. doi: 10.1007/BF00690207. [DOI] [PubMed] [Google Scholar]
- Kleibeuker J. H., van der Meer R., de Vries E. G. Calcium and vitamin D: possible protective agents against colorectal cancer? Eur J Cancer. 1995 Jul-Aug;31A(7-8):1081–1084. doi: 10.1016/0959-8049(95)00135-6. [DOI] [PubMed] [Google Scholar]
- Levin B. The role of dietary factors and chemoprevention in gastrointestinal malignancy. Curr Opin Oncol. 1995 Jul;7(4):377–380. doi: 10.1097/00001622-199507000-00015. [DOI] [PubMed] [Google Scholar]
- Lipkin M., Enker W. E., Winawer S. J. Tritiated-thymidine labeling of rectal epithelial cells in 'non-prep' biopsies of individuals at increased risk for colonic neoplasia. Cancer Lett. 1987 Oct 30;37(2):153–161. doi: 10.1016/0304-3835(87)90158-3. [DOI] [PubMed] [Google Scholar]
- Lipkin M., Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985 Nov 28;313(22):1381–1384. doi: 10.1056/NEJM198511283132203. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Phase 1 and phase 2 proliferative lesions of colonic epithelial cells in diseases leading to colonic cancer. Cancer. 1974 Sep;34(3):suppl–suppl:888. doi: 10.1002/1097-0142(197409)34:3+<878::aid-cncr2820340715>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Lupton J. R., Chen X. Q., Frølich W., Schoeffler G. L., Peterson M. L. Rats fed high fat diets with increased calcium levels have fecal bile acid concentrations similar to those of rats fed a low fat diet. J Nutr. 1994 Feb;124(2):188–195. doi: 10.1093/jn/124.2.188. [DOI] [PubMed] [Google Scholar]
- Lupton J. R., Steinbach G., Chang W. C., O'Brien B. C., Wiese S., Stoltzfus C. L., Glober G. A., Wargovich M. J., McPherson R. S., Winn R. J. Calcium supplementation modifies the relative amounts of bile acids in bile and affects key aspects of human colon physiology. J Nutr. 1996 May;126(5):1421–1428. doi: 10.1093/jn/126.5.1421. [DOI] [PubMed] [Google Scholar]
- Newmark H. L., Lipkin M., Maheshwari N. Colonic hyperproliferation induced in rats and mice by nutritional-stress diets containing four components of a human Western-style diet (series 2). Am J Clin Nutr. 1991 Jul;54(1 Suppl):209S–214S. doi: 10.1093/ajcn/54.1.209S. [DOI] [PubMed] [Google Scholar]
- Newmark H. L., Wargovich M. J., Bruce W. R. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984 Jun;72(6):1323–1325. [PubMed] [Google Scholar]
- Nobre-Leitão C., Chaves P., Fidalgo P., Cravo M., Gouveia-Oliveira A., Ferra M. A., Mira F. C. Calcium regulation of colonic crypt cell kinetics: evidence for a direct effect in mice. Gastroenterology. 1995 Aug;109(2):498–504. doi: 10.1016/0016-5085(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Patchett S. E., Alstead E. M., Saunders B. P., Hodgson S. V., Farthing M. J. Regional proliferative patterns in the colon of patients at risk for hereditary nonpolyposis colorectal cancer. Dis Colon Rectum. 1997 Feb;40(2):168–171. doi: 10.1007/BF02054982. [DOI] [PubMed] [Google Scholar]
- Pazianas M., Adebanjo O. A., Shankar V. S., James S. Y., Colston K. W., Maxwell J. D., Zaidi M. Extracellular cation sensing by the enterocyte: prediction of a novel divalent cation "receptor". Biochem Biophys Res Commun. 1995 May 25;210(3):948–953. doi: 10.1006/bbrc.1995.1748. [DOI] [PubMed] [Google Scholar]
- Pence B. C., Dunn D. M., Zhao C., Landers M., Wargovich M. J. Chemopreventive effects of calcium but not aspirin supplementation in cholic acid-promoted colon carcinogenesis: correlation with intermediate endpoints. Carcinogenesis. 1995 Apr;16(4):757–765. doi: 10.1093/carcin/16.4.757. [DOI] [PubMed] [Google Scholar]
- Pence B. C. Role of calcium in colon cancer prevention: experimental and clinical studies. Mutat Res. 1993 Nov;290(1):87–95. doi: 10.1016/0027-5107(93)90036-f. [DOI] [PubMed] [Google Scholar]
- Richter F., Newmark H. L., Richter A., Leung D., Lipkin M. Inhibition of Western-diet induced hyperproliferation and hyperplasia in mouse colon by two sources of calcium. Carcinogenesis. 1995 Nov;16(11):2685–2689. doi: 10.1093/carcin/16.11.2685. [DOI] [PubMed] [Google Scholar]
- Rooney P. S., Clarke P. A., Gifford K. A., Hardcastle J. D., Armitage N. C. The identification of high and low risk groups for colorectal cancer using rectal mucosal crypt cell production rate (CCPR). Br J Cancer. 1993 Jul;68(1):172–175. doi: 10.1038/bjc.1993.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen P., Fireman Z., Fine N., Wax Y., Ron E. Oral calcium suppresses increased rectal epithelial proliferation of persons at risk of colorectal cancer. Gut. 1989 May;30(5):650–655. doi: 10.1136/gut.30.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Ahnen D. J. Regional variability of colonocyte growth and differentiation in the rat. Anat Rec. 1992 Jul;233(3):409–414. doi: 10.1002/ar.1092330308. [DOI] [PubMed] [Google Scholar]
- Slattery M. L., Sorenson A. W., Ford M. H. Dietary calcium intake as a mitigating factor in colon cancer. Am J Epidemiol. 1988 Sep;128(3):504–514. doi: 10.1093/oxfordjournals.aje.a114998. [DOI] [PubMed] [Google Scholar]
- Stemmermann G. N., Nomura A., Chyou P. H. The influence of dairy and nondairy calcium on subsite large-bowel cancer risk. Dis Colon Rectum. 1990 Mar;33(3):190–194. doi: 10.1007/BF02134177. [DOI] [PubMed] [Google Scholar]
- Thomas M. G., Thomson J. P., Williamson R. C. Oral calcium inhibits rectal epithelial proliferation in familial adenomatous polyposis. Br J Surg. 1993 Apr;80(4):499–501. doi: 10.1002/bjs.1800800432. [DOI] [PubMed] [Google Scholar]
- Wargovich M. J., Allnutt D., Palmer C., Anaya P., Stephens L. C. Inhibition of the promotional phase of azoxymethane-induced colon carcinogenesis in the F344 rat by calcium lactate: effect of simulating two human nutrient density levels. Cancer Lett. 1990 Aug;53(1):17–25. doi: 10.1016/0304-3835(90)90005-i. [DOI] [PubMed] [Google Scholar]
- Weisgerber U. M., Boeing H., Owen R. W., Waldherr R., Raedsch R., Wahrendorf J. Effect of longterm placebo controlled calcium supplementation on sigmoidal cell proliferation in patients with sporadic adenomatous polyps. Gut. 1996 Mar;38(3):396–402. doi: 10.1136/gut.38.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. F., Bird R. P., Chakravarthy B. R., Isaacs R. J., Morley P. Calcium-cell cycle regulator, differentiator, killer, chemopreventor, and maybe, tumor promoter. J Cell Biochem Suppl. 1995;22:74–91. [PubMed] [Google Scholar]
- Wilson R. G., Smith A. N., Bird C. C. Immunohistochemical detection of abnormal cell proliferation in colonic mucosa of subjects with polyps. J Clin Pathol. 1990 Sep;43(9):744–747. doi: 10.1136/jcp.43.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]


