Abstract
This leader reviews recent advances in immunohistochemistry that are useful in the diagnosis of ovarian neoplasms. These include the value of different anticytokeratin antibodies in the distinction between a primary ovarian adenocarcinoma and a metastatic adenocarcinoma, especially of colorectal origin. These antibodies have also helped to clarify the origin of the peritoneal disease in most cases of pseudomyxoma peritonei. The value of antibodies against so called tumour specific antigens, such as CA125 and HAM56, in determining the ovarian origin of an adenocarcinoma is also reviewed. In recent years, several studies have investigated the value of a variety of monoclonal antibodies in the diagnosis of ovarian sex cord stromal tumours and in the distinction between these neoplasms and their histological mimics. These antibodies include those directed against inhibin, CD99, Mullerian inhibiting substance, relaxin like factor, melan A, and calretinin. Of these, anti-α inhibin appears to be of most diagnostic value. It is stressed that these antibodies should always be used as part of a larger panel and not in isolation.J Clin Pathol(J Clin Pathol 2000;53:327–334)
Key Words: ovarian neoplasms • diagnosis • immunohistochemistry
Full Text
The Full Text of this article is available as a PDF (224.6 KB).
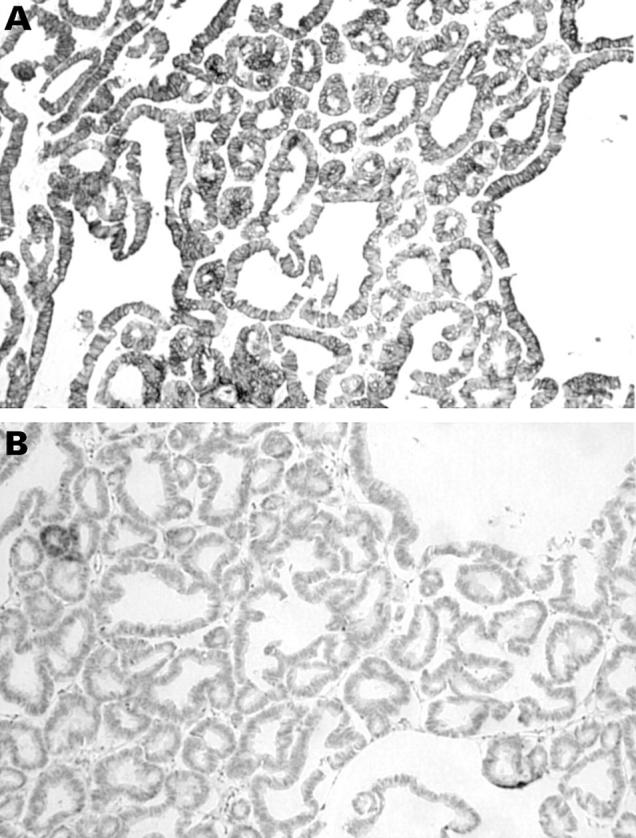
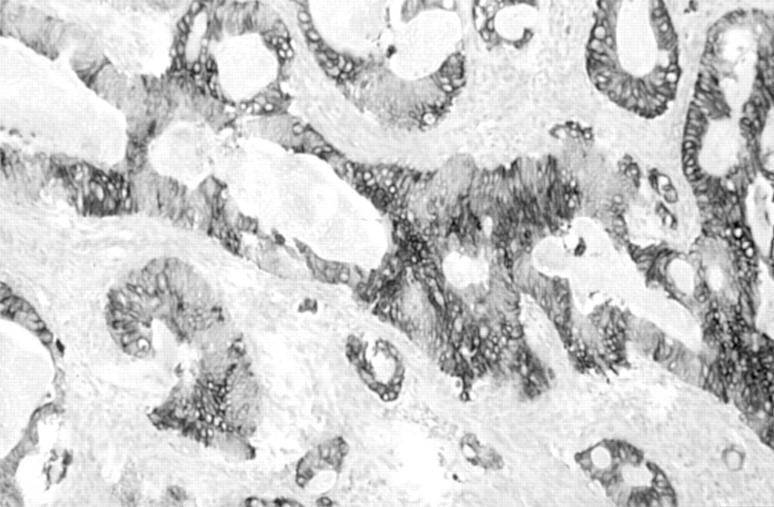
Figure 1 (A) Primary ovarian mucinous adenocarcinoma in which there is strong positive staining for cytokeratin 7 (CK7). (B) Same primary ovarian mucinous adenocarcinoma showing no staining for CK20.
Figure 2 Metastatic colonic adenocarcinoma in ovary exhibiting strong positive staining for cytokeratin 20 (CK20).
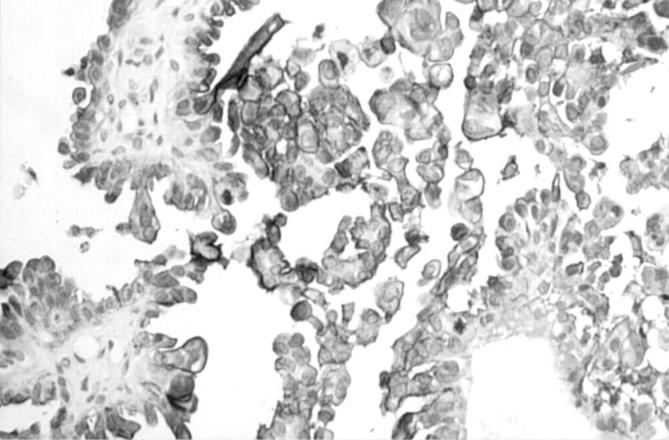
Figure 3 Strong positive staining of ovarian serous adenocarcinoma for CA125.
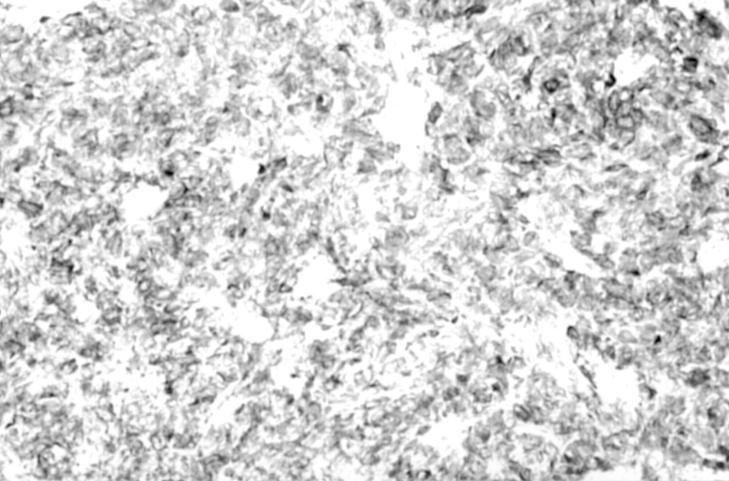
Figure 4 Strong positive staining of adult granulosa cell tumour for α inhibin.
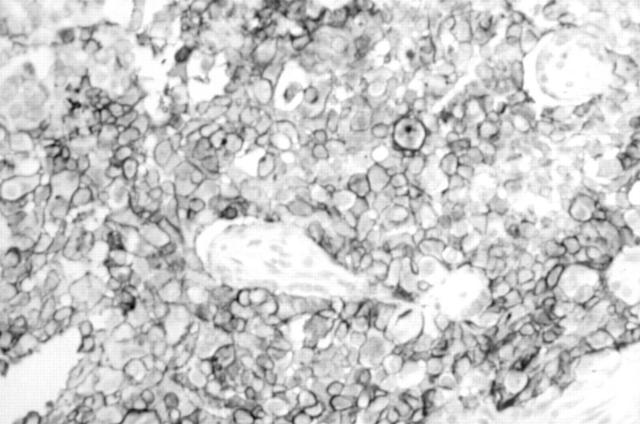
Figure 5 Strong membrane staining of a juvenile granulosa cell tumour with monoclonal antibody 013 (anti-CD99).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre P., Thor A. D., Scully R. E. Ovarian small cell carcinoma. Histogenetic considerations based on immunohistochemical and other findings. Am J Clin Pathol. 1989 Aug;92(2):140–149. doi: 10.1093/ajcp/92.2.140. [DOI] [PubMed] [Google Scholar]
- Ahmed E., Young R. H., Scully R. E. Adult granulosa cell tumor of the ovary with foci of hepatic cell differentiation: a report of four cases and comparison with two cases of granulosa cell tumor with Leydig cells. Am J Surg Pathol. 1999 Sep;23(9):1089–1093. doi: 10.1097/00000478-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Ambros I. M., Ambros P. F., Strehl S., Kovar H., Gadner H., Salzer-Kuntschik M. MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991 Apr 1;67(7):1886–1893. doi: 10.1002/1097-0142(19910401)67:7<1886::aid-cncr2820670712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Arora D. S., Cooke I. E., Ganesan T. S., Ramsdale J., Manek S., Charnock F. M., Groome N. P., Wells M. Immunohistochemical expression of inhibin/activin subunits in epithelial and granulosa cell tumours of the ovary. J Pathol. 1997 Apr;181(4):413–418. doi: 10.1002/(SICI)1096-9896(199704)181:4<413::AID-PATH789>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Arora D. S., Cooke I. E., Ganesan T. S., Ramsdale J., Manek S., Charnock F. M., Groome N. P., Wells M. Immunohistochemical expression of inhibin/activin subunits in epithelial and granulosa cell tumours of the ovary. J Pathol. 1997 Apr;181(4):413–418. doi: 10.1002/(SICI)1096-9896(199704)181:4<413::AID-PATH789>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Baker R. J., Hildebrandt R. H., Rouse R. V., Hendrickson M. R., Longacre T. A. Inhibin and CD99 (MIC2) expression in uterine stromal neoplasms with sex-cord-like elements. Hum Pathol. 1999 Jun;30(6):671–679. doi: 10.1016/s0046-8177(99)90093-x. [DOI] [PubMed] [Google Scholar]
- Bamberger A. M., Ivell R., Balvers M., Kelp B., Bamberger C. M., Riethdorf L., Löning T. Relaxin-like factor (RLF): a new specific marker for Leydig cells in the ovary. Int J Gynecol Pathol. 1999 Apr;18(2):163–168. [PubMed] [Google Scholar]
- Bateman A. C., al-Talib R. K., Newman T., Williams J. H., Herbert A. Immunohistochemical phenotype of malignant mesothelioma: predictive value of CA125 and HBME-1 expression. Histopathology. 1997 Jan;30(1):49–56. doi: 10.1046/j.1365-2559.1996.d01-562.x. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Finegold M. J., Cate R. L. Müllerian-inhibiting substance function during mammalian sexual development. Cell. 1994 Nov 4;79(3):415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Berezowski K., Stastny J. F., Kornstein M. J. Cytokeratins 7 and 20 and carcinoembryonic antigen in ovarian and colonic carcinoma. Mod Pathol. 1996 Apr;9(4):426–429. [PubMed] [Google Scholar]
- Blessing K., Sanders D. S., Grant J. J. Comparison of immunohistochemical staining of the novel antibody melan-A with S100 protein and HMB-45 in malignant melanoma and melanoma variants. Histopathology. 1998 Feb;32(2):139–146. doi: 10.1046/j.1365-2559.1998.00312.x. [DOI] [PubMed] [Google Scholar]
- Buchanan CD, Chun SB. Simple predictive model for flavor production in hadronization. Phys Rev Lett. 1987 Nov 2;59(18):1997–2000. doi: 10.1103/PhysRevLett.59.1997. [DOI] [PubMed] [Google Scholar]
- Buist M. R., Molthoff C. F., Kenemans P., Meijer C. J. Distribution of OV-TL 3 and MOv18 in normal and malignant ovarian tissue. J Clin Pathol. 1995 Jul;48(7):631–636. doi: 10.1136/jcp.48.7.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busam K. J., Iversen K., Coplan K. A., Old L. J., Stockert E., Chen Y. T., McGregor D., Jungbluth A. Immunoreactivity for A103, an antibody to melan-A (Mart-1), in adrenocortical and other steroid tumors. Am J Surg Pathol. 1998 Jan;22(1):57–63. doi: 10.1097/00000478-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Cheung A. N., Chiu P. M., Khoo U. S. Is immunostaining with HAM56 antibody useful in identifying ovarian origin of metastatic adenocarcinomas? Hum Pathol. 1997 Jan;28(1):91–94. doi: 10.1016/s0046-8177(97)90285-9. [DOI] [PubMed] [Google Scholar]
- Chuaqui R. F., Zhuang Z., Emmert-Buck M. R., Bryant B. R., Nogales F., Tavassoli F. A., Merino M. J. Genetic analysis of synchronous mucinous tumors of the ovary and appendix. Hum Pathol. 1996 Feb;27(2):165–171. doi: 10.1016/s0046-8177(96)90370-6. [DOI] [PubMed] [Google Scholar]
- Cooke I., O'Brien M., Charnock F. M., Groome N., Ganesan T. S. Inhibin as a marker for ovarian cancer. Br J Cancer. 1995 May;71(5):1046–1050. doi: 10.1038/bjc.1995.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. J., Ames P. F., Walls J., Roth L. M. Inhibin immunohistochemistry applied to ovarian neoplasms: a novel, effective, diagnostic tool. Hum Pathol. 1997 Nov;28(11):1247–1254. doi: 10.1016/s0046-8177(97)90197-0. [DOI] [PubMed] [Google Scholar]
- Costa M. J., DeRose P. B., Roth L. M., Brescia R. J., Zaloudek C. J., Cohen C. Immunohistochemical phenotype of ovarian granulosa cell tumors: absence of epithelial membrane antigen has diagnostic value. Hum Pathol. 1994 Jan;25(1):60–66. doi: 10.1016/0046-8177(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Crawford R. J., Hammond V. E., Evans B. A., Coghlan J. P., Haralambidis J., Hudson B., Penschow J. D., Richards R. I., Tregear G. W. Alpha-inhibin gene expression occurs in the ovine adrenal cortex, and is regulated by adrenocorticotropin. Mol Endocrinol. 1987 Oct;1(10):699–706. doi: 10.1210/mend-1-10-699. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas M., Matias-Guiu X., Prat J. Synchronous mucinous tumors of the appendix and the ovary associated with pseudomyxoma peritonei. A clinicopathologic study of six cases with comparative analysis of c-Ki-ras mutations. Am J Surg Pathol. 1996 Jun;20(6):739–746. doi: 10.1097/00000478-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Daya D., Nazerali L., Frank G. L. Metastatic ovarian carcinoma of large intestinal origin simulating primary ovarian carcinoma. A clinicopathologic study of 25 cases. Am J Clin Pathol. 1992 Jun;97(6):751–758. doi: 10.1093/ajcp/97.6.751. [DOI] [PubMed] [Google Scholar]
- Demopoulos R. I., Sitelman A., Flotte T., Bigelow B. Ultrastructural study of a female adnexal tumor of probable wolffian origin. Cancer. 1980 Nov 15;46(10):2273–2280. doi: 10.1002/1097-0142(19801115)46:10<2273::aid-cncr2820461027>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Devouassoux-Shisheboran M., Silver S. A., Tavassoli F. A. Wolffian adnexal tumor, so-called female adnexal tumor of probable Wolffian origin (FATWO): immunohistochemical evidence in support of a Wolffian origin. Hum Pathol. 1999 Jul;30(7):856–863. doi: 10.1016/s0046-8177(99)90148-x. [DOI] [PubMed] [Google Scholar]
- Doglioni C., Dei Tos A. P., Laurino L., Iuzzolino P., Chiarelli C., Celio M. R., Viale G. Calretinin: a novel immunocytochemical marker for mesothelioma. Am J Surg Pathol. 1996 Sep;20(9):1037–1046. doi: 10.1097/00000478-199609000-00001. [DOI] [PubMed] [Google Scholar]
- Flemming P., Grothe W., Maschek H., Petry K. U., Wellmann A., Georgii A. The site of inhibin production in ovarian neoplasms. Histopathology. 1996 Nov;29(5):465–468. doi: 10.1046/j.1365-2559.1996.d01-511.x. [DOI] [PubMed] [Google Scholar]
- Flemming P., Wellmann A., Maschek H., Lang H., Georgii A. Monoclonal antibodies against inhibin represent key markers of adult granulosa cell tumors of the ovary even in their metastases. A report of three cases with late metastasis, being previously misinterpreted as hemangiopericytoma. Am J Surg Pathol. 1995 Aug;19(8):927–933. doi: 10.1097/00000478-199508000-00008. [DOI] [PubMed] [Google Scholar]
- Fowler L. J., Maygarden S. J., Novotny D. B. Human alveolar macrophage-56 and carcinoembryonic antigen monoclonal antibodies in the differential diagnosis between primary ovarian and metastatic gastrointestinal carcinomas. Hum Pathol. 1994 Jul;25(7):666–670. doi: 10.1016/0046-8177(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Gagnon S., Têtu B., Silva E. G., McCaughey W. T. Frequency of alpha-fetoprotein production by Sertoli-Leydig cell tumors of the ovary: an immunohistochemical study of eight cases. Mod Pathol. 1989 Jan;2(1):63–67. [PubMed] [Google Scholar]
- Gordon M. D., Corless C., Renshaw A. A., Beckstead J. CD99, keratin, and vimentin staining of sex cord-stromal tumors, normal ovary, and testis. Mod Pathol. 1998 Aug;11(8):769–773. [PubMed] [Google Scholar]
- Guerrieri C., Frånlund B., Fristedt S., Gillooley J. F., Boeryd B. Mucinous tumors of the vermiform appendix and ovary, and pseudomyxoma peritonei: histogenetic implications of cytokeratin 7 expression. Hum Pathol. 1997 Sep;28(9):1039–1045. doi: 10.1016/s0046-8177(97)90057-5. [DOI] [PubMed] [Google Scholar]
- Guerrieri C., Frånlund B., Malmström H., Boeryd B. Ovarian endometrioid carcinomas simulating sex cord-stromal tumors: a study using inhibin and cytokeratin 7. Int J Gynecol Pathol. 1998 Jul;17(3):266–271. doi: 10.1097/00004347-199807000-00012. [DOI] [PubMed] [Google Scholar]
- Gustafson M. L., Lee M. M., Scully R. E., Moncure A. C., Hirakawa T., Goodman A., Muntz H. G., Donahoe P. K., MacLaughlin D. T., Fuller A. F., Jr Müllerian inhibiting substance as a marker for ovarian sex-cord tumor. N Engl J Med. 1992 Feb 13;326(7):466–471. doi: 10.1056/NEJM199202133260707. [DOI] [PubMed] [Google Scholar]
- Hammad A., Jasnosz K. M., Olson P. R. Expression of alpha-fetoprotein by ovarian Sertoli-Leydig cell tumors. Case report and review of the literature. Arch Pathol Lab Med. 1995 Nov;119(11):1075–1079. [PubMed] [Google Scholar]
- Healy D. L., Burger H. G., Mamers P., Jobling T., Bangah M., Quinn M., Grant P., Day A. J., Rome R., Campbell J. J. Elevated serum inhibin concentrations in postmenopausal women with ovarian tumors. N Engl J Med. 1993 Nov 18;329(21):1539–1542. doi: 10.1056/NEJM199311183292104. [DOI] [PubMed] [Google Scholar]
- Henzen-Logmans S. C., Schipper N. W., Poels L. G., Stolk K., Kenemans P., Meyer C. J. Use of statistical evaluation of antigen profiles in differential diagnosis between colonic and ovarian adenocarcinomas. J Clin Pathol. 1988 Jun;41(6):644–649. doi: 10.1136/jcp.41.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt R. H., Rouse R. V., Longacre T. A. Value of inhibin in the identification of granulosa cell tumors of the ovary. Hum Pathol. 1997 Dec;28(12):1387–1395. doi: 10.1016/s0046-8177(97)90229-x. [DOI] [PubMed] [Google Scholar]
- Hussong J., Crussi F. G., Chou P. M. Gonadoblastoma: immunohistochemical localization of Müllerian-inhibiting substance, inhibin, WT-1, and p53. Mod Pathol. 1997 Nov;10(11):1101–1105. [PubMed] [Google Scholar]
- Ivell R. Biology of the relaxin-like factor (RLF). Rev Reprod. 1997 Sep;2(3):133–138. doi: 10.1530/ror.0.0020133. [DOI] [PubMed] [Google Scholar]
- Jacobs I., Bast R. C., Jr The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989 Jan;4(1):1–12. doi: 10.1093/oxfordjournals.humrep.a136832. [DOI] [PubMed] [Google Scholar]
- Kawauchi S., Fukuda T., Miyamoto S., Yoshioka J., Shirahama S., Saito T., Tsukamoto N. Peripheral primitive neuroectodermal tumor of the ovary confirmed by CD99 immunostaining, karyotypic analysis, and RT-PCR for EWS/FLI-1 chimeric mRNA. Am J Surg Pathol. 1998 Nov;22(11):1417–1422. doi: 10.1097/00000478-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Kommoss F., Oliva E., Bhan A. K., Young R. H., Scully R. E. Inhibin expression in ovarian tumors and tumor-like lesions: an immunohistochemical study. Mod Pathol. 1998 Jul;11(7):656–664. [PubMed] [Google Scholar]
- Koonings P. P., Campbell K., Mishell D. R., Jr, Grimes D. A. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989 Dec;74(6):921–926. [PubMed] [Google Scholar]
- Krishnamurthy S., Jungbluth A. A., Busam K. J., Rosai J. Uterine tumors resembling ovarian sex-cord tumors have an immunophenotype consistent with true sex-cord differentiation. Am J Surg Pathol. 1998 Sep;22(9):1078–1082. doi: 10.1097/00000478-199809000-00006. [DOI] [PubMed] [Google Scholar]
- Kuroda T., Lee M. M., Ragin R. C., Hirobe S., Donahoe P. K. Müllerian inhibiting substance production and cleavage is modulated by gonadotropins and steroids. Endocrinology. 1991 Dec;129(6):2985–2992. doi: 10.1210/endo-129-6-2985. [DOI] [PubMed] [Google Scholar]
- Laeng R. H., Stamm B. Kikuchi's histiocytic necrotizing lymphadenitis driven by activated cytolytic T-cells: an example associated with systemic scleroderma. Histopathology. 1999 Apr;34(4):373–374. doi: 10.1046/j.1365-2559.1999.0669a.x. [DOI] [PubMed] [Google Scholar]
- Lagendijk J. H., Mullink H., Van Diest P. J., Meijer G. A., Meijer C. J. Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry: differential diagnosis between colonic and ovarian carcinomas as primary sites. Hum Pathol. 1998 May;29(5):491–497. doi: 10.1016/s0046-8177(98)90065-x. [DOI] [PubMed] [Google Scholar]
- Lappöhn R. E., Burger H. G., Bouma J., Bangah M., Krans M., de Bruijn H. W. Inhibin as a marker for granulosa-cell tumors. N Engl J Med. 1989 Sep 21;321(12):790–793. doi: 10.1056/NEJM198909213211204. [DOI] [PubMed] [Google Scholar]
- Lash R. H., Hart W. R. Intestinal adenocarcinomas metastatic to the ovaries. A clinicopathologic evaluation of 22 cases. Am J Surg Pathol. 1987 Feb;11(2):114–121. doi: 10.1097/00000478-198702000-00005. [DOI] [PubMed] [Google Scholar]
- Loo K. T., Leung A. K., Chan J. K. Immunohistochemical staining of ovarian granulosa cell tumours with MIC2 antibody. Histopathology. 1995 Oct;27(4):388–390. doi: 10.1111/j.1365-2559.1995.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Loy T. S., Abshier J. Immunostaining with HAM56 in the diagnosis of adenocarcinomas. Mod Pathol. 1993 Jul;6(4):473–475. [PubMed] [Google Scholar]
- Loy T. S., Calaluce R. D., Keeney G. L. Cytokeratin immunostaining in differentiating primary ovarian carcinoma from metastatic colonic adenocarcinoma. Mod Pathol. 1996 Nov;9(11):1040–1044. [PubMed] [Google Scholar]
- Loy T. S., Quesenberry J. T., Sharp S. C. Distribution of CA 125 in adenocarcinomas. An immunohistochemical study of 481 cases. Am J Clin Pathol. 1992 Aug;98(2):175–179. doi: 10.1093/ajcp/98.2.175. [DOI] [PubMed] [Google Scholar]
- Maida Y., Kyo S., Takakura M., Kanaya T., Inoue M. Ovarian endometrioid adenocarcinoma with ectopic production of alpha-fetoprotein. Gynecol Oncol. 1998 Oct;71(1):133–136. doi: 10.1006/gyno.1998.5119. [DOI] [PubMed] [Google Scholar]
- Matias-Guiu X., Pons C., Prat J. Müllerian inhibiting substance, alpha-inhibin, and CD99 expression in sex cord-stromal tumors and endometrioid ovarian carcinomas resembling sex cord-stromal tumors. Hum Pathol. 1998 Aug;29(8):840–845. doi: 10.1016/s0046-8177(98)90454-3. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Ashe P., McBride H., Maxwell P., Sloan J. M. Localization of the cellular expression of inhibin in trophoblastic tissue. Histopathology. 1998 Mar;32(3):252–256. doi: 10.1046/j.1365-2559.1998.00385.x. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Burton J., Maxwell P., Sloan J. M. Immunohistochemical staining of normal, hyperplastic, and neoplastic adrenal cortex with a monoclonal antibody against alpha inhibin. J Clin Pathol. 1998 Feb;51(2):114–116. doi: 10.1136/jcp.51.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluggage W. G., Maxwell P. Adenocarcinomas of various sites may exhibit immunoreactivity with anti-inhibin antibodies. Histopathology. 1999 Sep;35(3):216–220. doi: 10.1046/j.1365-2559.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Maxwell P., Patterson A., Sloan J. M. Immunohistochemical staining of hepatocellular carcinoma with monoclonal antibody against inhibin. Histopathology. 1997 Jun;30(6):518–522. doi: 10.1046/j.1365-2559.1997.5580774.x. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Maxwell P., Sloan J. M. Immunohistochemical staining of ovarian granulosa cell tumors with monoclonal antibody against inhibin. Hum Pathol. 1997 Sep;28(9):1034–1038. doi: 10.1016/s0046-8177(97)90056-3. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Patterson A., White J., Anderson N. H. Immunocytochemical staining of ovarian cyst aspirates with monoclonal antibody against inhibin. Cytopathology. 1998 Oct;9(5):336–342. doi: 10.1046/j.1365-2303.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Shanks J. H., Whiteside C., Maxwell P., Banerjee S. S., Biggart J. D. Immunohistochemical study of testicular sex cord-stromal tumors, including staining with anti-inhibin antibody. Am J Surg Pathol. 1998 May;22(5):615–619. doi: 10.1097/00000478-199805000-00013. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Sloan J. M., Boyle D. D., Toner P. G. Malignant fibrothecomatous tumour of the ovary: diagnostic value of anti-inhibin immunostaining. J Clin Pathol. 1998 Nov;51(11):868–871. doi: 10.1136/jcp.51.11.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluggage W. G., Sloan J. M., Murnaghan M., White R. Gynandroblastoma of ovary with juvenile granulosa cell component and heterologous intestinal type glands. Histopathology. 1996 Sep;29(3):253–257. doi: 10.1111/j.1365-2559.1996.tb01399.x. [DOI] [PubMed] [Google Scholar]
- McLachlan R. I., Robertson D. M., Healy D. L., Burger H. G., de Kretser D. M. Circulating immunoreactive inhibin levels during the normal human menstrual cycle. J Clin Endocrinol Metab. 1987 Nov;65(5):954–961. doi: 10.1210/jcem-65-5-954. [DOI] [PubMed] [Google Scholar]
- Meunier H., Rivier C., Evans R. M., Vale W. Gonadal and extragonadal expression of inhibin alpha, beta A, and beta B subunits in various tissues predicts diverse functions. Proc Natl Acad Sci U S A. 1988 Jan;85(1):247–251. doi: 10.1073/pnas.85.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney E. E., Nogales F. F., Tavassoli F. A. Hepatocytic differentiation in retiform Sertoli-Leydig cell tumors: distinguishing a heterologous element from Leydig cells. Hum Pathol. 1999 Jun;30(6):611–617. doi: 10.1016/s0046-8177(99)90083-7. [DOI] [PubMed] [Google Scholar]
- Nap M., Hammarström M. L., Börmer O., Hammarström S., Wagener C., Handt S., Schreyer M., Mach J. P., Buchegger F., von Kleist S. Specificity and affinity of monoclonal antibodies against carcinoembryonic antigen. Cancer Res. 1992 Apr 15;52(8):2329–2339. [PubMed] [Google Scholar]
- Nouwen E. J., Pollet D. E., Eerdekens M. W., Hendrix P. G., Briers T. W., De Broe M. E. Immunohistochemical localization of placental alkaline phosphatase, carcinoembryonic antigen, and cancer antigen 125 in normal and neoplastic human lung. Cancer Res. 1986 Feb;46(2):866–876. [PubMed] [Google Scholar]
- Nucci M. R., Krausz T., Lifschitz-Mercer B., Chan J. K., Fletcher C. D. Angiosarcoma of the ovary: clinicopathologic and immunohistochemical analysis of four cases with a broad morphologic spectrum. Am J Surg Pathol. 1998 May;22(5):620–630. doi: 10.1097/00000478-199805000-00014. [DOI] [PubMed] [Google Scholar]
- Otis C. N., Powell J. L., Barbuto D., Carcangiu M. L. Intermediate filamentous proteins in adult granulosa cell tumors. An immunohistochemical study of 25 cases. Am J Surg Pathol. 1992 Oct;16(10):962–968. doi: 10.1097/00000478-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Pavelic Z. P., Petrelli N. J., Herrera L., Vaughan M. M., Paecock J. S., Pavelic L. D-14 monoclonal antibody to carcinoembryonic antigen: immunohistochemical analysis of formalin-fixed, paraffin-embedded human colorectal carcinoma, tumors of non-colorectal origin and normal tissues. J Cancer Res Clin Oncol. 1990;116(1):51–56. doi: 10.1007/BF01612640. [DOI] [PubMed] [Google Scholar]
- Pelkey T. J., Frierson H. F., Jr, Mills S. E., Stoler M. H. The diagnostic utility of inhibin staining in ovarian neoplasms. Int J Gynecol Pathol. 1998 Apr;17(2):97–105. doi: 10.1097/00004347-199804000-00001. [DOI] [PubMed] [Google Scholar]
- Prayson R. A., Hart W. R., Petras R. E. Pseudomyxoma peritonei. A clinicopathologic study of 19 cases with emphasis on site of origin and nature of associated ovarian tumors. Am J Surg Pathol. 1994 Jun;18(6):591–603. [PubMed] [Google Scholar]
- Riopel M. A., Perlman E. J., Seidman J. D., Kurman R. J., Sherman M. E. Inhibin and epithelial membrane antigen immunohistochemistry assist in the diagnosis of sex cord-stromal tumors and provide clues to the histogenesis of hypercalcemic small cell carcinomas. Int J Gynecol Pathol. 1998 Jan;17(1):46–53. doi: 10.1097/00004347-199801000-00009. [DOI] [PubMed] [Google Scholar]
- Rishi M., Howard L. N., Bratthauer G. L., Tavassoli F. A. Use of monoclonal antibody against human inhibin as a marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol. 1997 May;21(5):583–589. doi: 10.1097/00000478-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Ronnett B. M., Kurman R. J., Zahn C. M., Shmookler B. M., Jablonski K. A., Kass M. E., Sugarbaker P. H. Pseudomyxoma peritonei in women: a clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol. 1995 May;26(5):509–524. doi: 10.1016/0046-8177(95)90247-3. [DOI] [PubMed] [Google Scholar]
- Ronnett B. M., Shmookler B. M., Diener-West M., Sugarbaker P. H., Kurman R. J. Immunohistochemical evidence supporting the appendiceal origin of pseudomyxoma peritonei in women. Int J Gynecol Pathol. 1997 Jan;16(1):1–9. doi: 10.1097/00004347-199701000-00001. [DOI] [PubMed] [Google Scholar]
- Ronnett B. M., Zahn C. M., Kurman R. J., Kass M. E., Sugarbaker P. H., Shmookler B. M. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to "pseudomyxoma peritonei". Am J Surg Pathol. 1995 Dec;19(12):1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- Seidman J. D., Elsayed A. M., Sobin L. H., Tavassoli F. A. Association of mucinous tumors of the ovary and appendix. A clinicopathologic study of 25 cases. Am J Surg Pathol. 1993 Jan;17(1):22–34. doi: 10.1097/00000478-199301000-00003. [DOI] [PubMed] [Google Scholar]
- Sheahan K., O'Brien M. J., Burke B., Dervan P. A., O'Keane J. C., Gottlieb L. S., Zamcheck N. Differential reactivities of carcinoembryonic antigen (CEA) and CEA-related monoclonal and polyclonal antibodies in common epithelial malignancies. Am J Clin Pathol. 1990 Aug;94(2):157–164. doi: 10.1093/ajcp/94.2.157. [DOI] [PubMed] [Google Scholar]
- Silverman L. A., Gitelman S. E. Immunoreactive inhibin, müllerian inhibitory substance, and activin as biochemical markers for juvenile granulosa cell tumors. J Pediatr. 1996 Dec;129(6):918–921. doi: 10.1016/s0022-3476(96)70040-9. [DOI] [PubMed] [Google Scholar]
- Soslow R. A., Rouse R. V., Hendrickson M. R., Silva E. G., Longacre T. A. Transitional cell neoplasms of the ovary and urinary bladder: a comparative immunohistochemical analysis. Int J Gynecol Pathol. 1996 Jul;15(3):257–265. doi: 10.1097/00004347-199607000-00011. [DOI] [PubMed] [Google Scholar]
- Stewart C. J., Jeffers M. D., Kennedy A. Diagnostic value of inhibin immunoreactivity in ovarian gonadal stromal tumours and their histological mimics. Histopathology. 1997 Jul;31(1):67–74. doi: 10.1046/j.1365-2559.1997.5780819.x. [DOI] [PubMed] [Google Scholar]
- Ueda G., Sawada M., Ogawa H., Tanizawa O., Tsujimoto M. Immunohistochemical study of cytokeratin 7 for the differential diagnosis of adenocarcinomas in the ovary. Gynecol Oncol. 1993 Nov;51(2):219–223. doi: 10.1006/gyno.1993.1276. [DOI] [PubMed] [Google Scholar]
- Ulbright T. M., Roth L. M., Stehman F. B. Secondary ovarian neoplasia. A clinicopathologic study of 35 cases. Cancer. 1984 Mar 1;53(5):1164–1174. doi: 10.1002/1097-0142(19840301)53:5<1164::aid-cncr2820530523>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Wauters C. C., Smedts F., Gerrits L. G., Bosman F. T., Ramaekers F. C. Keratins 7 and 20 as diagnostic markers of carcinomas metastatic to the ovary. Hum Pathol. 1995 Aug;26(8):852–855. doi: 10.1016/0046-8177(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Weidner N., Tjoe J. Immunohistochemical profile of monoclonal antibody O13: antibody that recognizes glycoprotein p30/32MIC2 and is useful in diagnosing Ewing's sarcoma and peripheral neuroepithelioma. Am J Surg Pathol. 1994 May;18(5):486–494. [PubMed] [Google Scholar]
- Welshinger M., Yin B. W., Lloyd K. O. Initial immunochemical characterization of MX35 ovarian cancer antigen. Gynecol Oncol. 1997 Nov;67(2):188–192. doi: 10.1006/gyno.1997.4846. [DOI] [PubMed] [Google Scholar]
- Younes M., Katikaneni P. R., Lechago L. V., Lechago J. HAM56 antibody: a tool in the differential diagnosis between colorectal and gynecological malignancy. Mod Pathol. 1994 Apr;7(3):396–400. [PubMed] [Google Scholar]
- Young R. H., Gilks C. B., Scully R. E. Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol. 1991 May;15(5):415–429. doi: 10.1097/00000478-199105000-00001. [DOI] [PubMed] [Google Scholar]
- Young R. H., Perez-Atayde A. R., Scully R. E. Ovarian Sertoli-Leydig cell tumor with retiform and heterologous components. Report of a case with hepatocytic differentiation and elevated serum alpha-fetoprotein. Am J Surg Pathol. 1984 Sep;8(9):709–718. doi: 10.1097/00000478-198409000-00011. [DOI] [PubMed] [Google Scholar]
- Young R. H., Scully R. E. Ovarian sex cord-stromal tumors. Problems in differential diagnosis. Pathol Annu. 1988;23(Pt 1):237–296. [PubMed] [Google Scholar]
- Zheng W., Sung C. J., Hanna I., DePetris G., Lambert-Messerlian G., Steinhoff M., Lauchlan S. C. Alpha and beta subunits of inhibin/activin as sex cord-stromal differentiation markers. Int J Gynecol Pathol. 1997 Jul;16(3):263–271. doi: 10.1097/00004347-199707000-00012. [DOI] [PubMed] [Google Scholar]