Abstract
Aims—Investigation of the histopathological changes in prostatectomy specimens of patients with prostate cancer after high intensity focused ultrasound (HIFU) and identification of immunohistochemical markers for tissue damage after HIFU treatment.
Methods—Nine patients diagnosed with adenocarcinoma of the prostate underwent unilateral HIFU treatment seven to 12 days before radical prostatectomy. The prostatectomy specimens were analysed histologically. Immunohistochemical staining and electron microscopy were performed to characterise more subtle phenotypic changes.
Results—All prostatectomy specimens revealed well circumscribed HIFU lesions at the dorsal side of the prostate lobe treated. Most epithelial glands in the centre of the HIFU lesions revealed signs of necrosis. Glands without apparently necrotic features were also situated in the HIFU lesions, raising the question of whether lethal destruction had occurred. This epithelium reacted with antibodies to pancytokeratin, prostate specific antigen (PSA), and Ki67, but did not express cytokeratin 8, which is indicative of severe cellular damage. Ultrastructural examination revealed disintegration of cellular membranes and cytoplasmic organelles consistent with cell necrosis. HIFU treatment was incomplete at the ventral, lateral, and dorsal sides of the prostate lobe treated.
Conclusions—HIFU treatment induces a spectrum of morphological changes ranging from apparent light microscopic necrosis to more subtle ultrastructural cell damage. All HIFU lesions are marked by loss of cytokeratin 8. HIFU does not affect the whole area treated, leaving vital tissue at the ventral, lateral, and dorsal sides of the prostate.
Key Words: prostate cancer • high intensity focused ultrasound treatment
Full Text
The Full Text of this article is available as a PDF (158.7 KB).
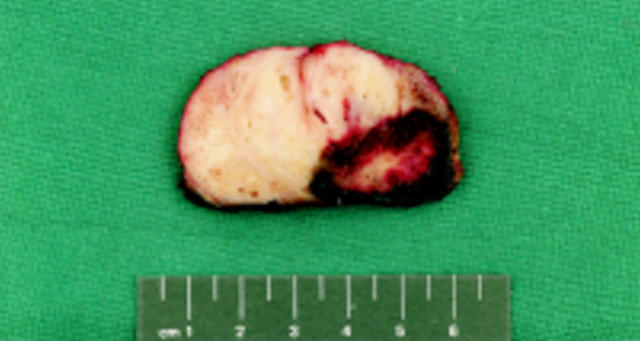
Figure 1 Prostate slice showing a high intensity focused ultrasound (HIFU) lesion. The HIFU lesion is characterised by a central yellow/white, necrotic zone surrounded by an outer dark red, haemorrhagic zone. The distance between two cross bars represents 0.5 cm.
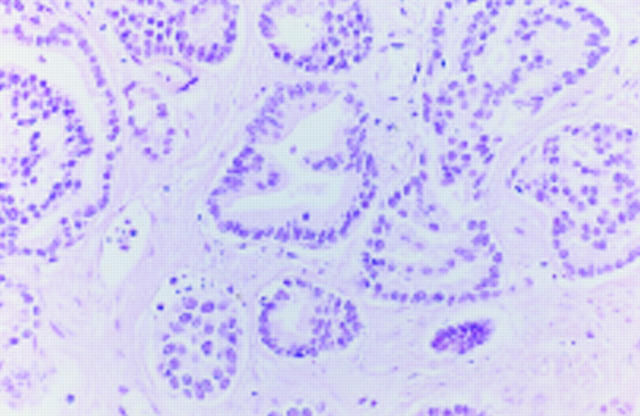
Figure 2 Adenocarcinoma in the centre of a high intensity focused ultrasound lesion without morphological signs of necrosis. Nuclear structures are well preserved and cell borders are locally identified.
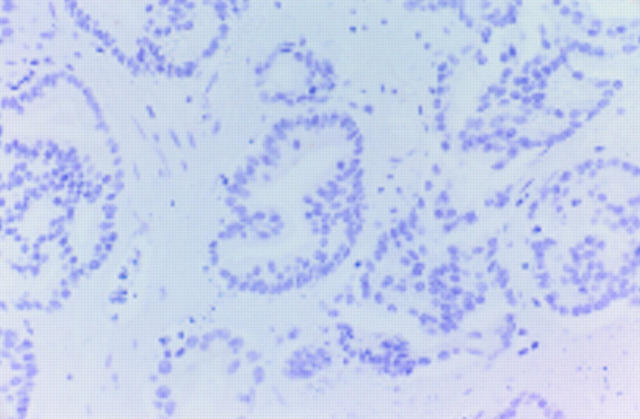
Figure 3 Weak expression of pancytokeratin in histomorpologically unaffected malignant epithelium within the high intensity focused ultrasound lesion. AE1/AE3 antibody.
Figure 4 Absence of cytokeratin 8 expression in histomorphologically unaffected malignant epithelium within the high intensity focused ultrasound lesion. CAM5.2 antibody.
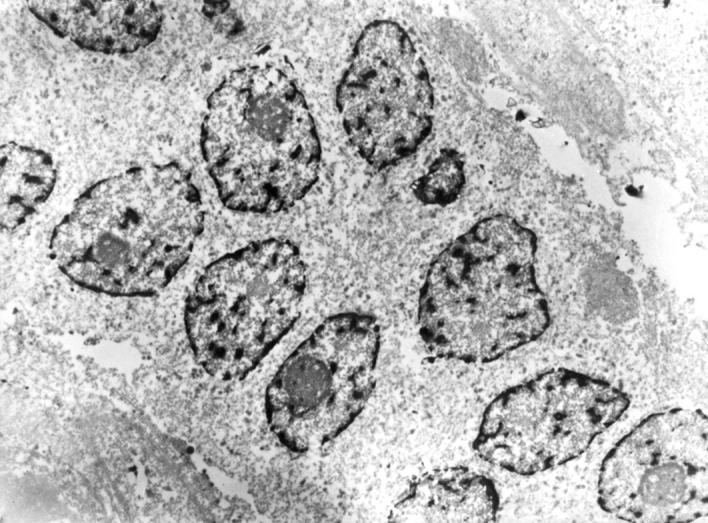
Figure 5 Electronmicrograph of an adenocarcinoma within the high intensity focused ultrasound lesion reveals destruction of cellular membranes and organelles but a relatively preserved nuclear structure. Magnification, x 3000.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beerlage H. P., van Leenders G. J., Oosterhof G. O., Witjes J. A., Ruijter E. T., van de Kaa C. A., Debruyne F. M., de la Rosette J. J. High-intensity focused ultrasound (HIFU) followed after one to two weeks by radical retropubic prostatectomy: results of a prospective study. Prostate. 1999 Apr 1;39(1):41–46. doi: 10.1002/(sici)1097-0045(19990401)39:1<41::aid-pros7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Bratt O., Elfving P., Flodgren P., Lundgren R. Morbidity of pelvic lymphadenectomy, radical retropubic prostatectomy and external radiotherapy in patients with localised prostatic cancer. Scand J Urol Nephrol. 1994 Sep;28(3):265–271. [PubMed] [Google Scholar]
- Ebert T., Graefen M., Miller S., Saddeler D., Schmitz-Dräger B., Ackermann R. High-intensity focused ultrasound (HIFU) in the treatment of benign prostatic hyperplasia (BPH). Keio J Med. 1995 Dec;44(4):146–149. doi: 10.2302/kjm.44.146. [DOI] [PubMed] [Google Scholar]
- Gelet A., Chapelon J. Y., Bouvier R., Pangaud C., Lasne Y. Local control of prostate cancer by transrectal high intensity focused ultrasound therapy: preliminary results. J Urol. 1999 Jan;161(1):156–162. [PubMed] [Google Scholar]
- Gelet A., Chapelon J. Y., Bouvier R., Souchon R., Pangaud C., Abdelrahim A. F., Cathignol D., Dubernard J. M. Treatment of prostate cancer with transrectal focused ultrasound: early clinical experience. Eur Urol. 1996;29(2):174–183. [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Ritchie K. P. Protein denaturation in intact hepatocytes and isolated cellular organelles during heat shock. J Cell Biol. 1993 Sep;122(6):1267–1276. doi: 10.1083/jcb.122.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madersbacher S., Klingler C. H., Schatzl G., Schmidbauer C. P., Marberger M. The urodynamic impact of transrectal high-intensity focused ultrasound on bladder outflow obstruction. Eur Urol. 1996;30(4):437–445. doi: 10.1159/000474212. [DOI] [PubMed] [Google Scholar]
- Madersbacher S., Pedevilla M., Vingers L., Susani M., Marberger M. Effect of high-intensity focused ultrasound on human prostate cancer in vivo. Cancer Res. 1995 Aug 1;55(15):3346–3351. [PubMed] [Google Scholar]
- Mulligan E. D., Lynch T. H., Mulvin D., Greene D., Smith J. M., Fitzpatrick J. M. High-intensity focused ultrasound in the treatment of benign prostatic hyperplasia. Br J Urol. 1997 Feb;79(2):177–180. doi: 10.1046/j.1464-410x.1997.03286.x. [DOI] [PubMed] [Google Scholar]
- Overgaard K., Overgaard J. Hyperthermic tumour-cell devitalization in vivo. Acta Radiol Ther Phys Biol. 1977 May;16(1):1–16. doi: 10.3109/02841867709133928. [DOI] [PubMed] [Google Scholar]
- Pisters L. L., von Eschenbach A. C., Scott S. M., Swanson D. A., Dinney C. P., Pettaway C. A., Babaian R. J. The efficacy and complications of salvage cryotherapy of the prostate. J Urol. 1997 Mar;157(3):921–925. [PubMed] [Google Scholar]
- Schröder F. H., Hermanek P., Denis L., Fair W. R., Gospodarowicz M. K., Pavone-Macaluso M. The TNM classification of prostate cancer. Prostate Suppl. 1992;4:129–138. doi: 10.1002/pros.2990210521. [DOI] [PubMed] [Google Scholar]
- Schwarzer J. U., Hofmann R., Kneschaurek P., Lukas P., Lindner A., Braun J. High-dose-rate-Brachytherapie des Prostatakarzinoms mit Iridium 192. Strahlenther Onkol. 1992 Jan;168(1):17–22. [PubMed] [Google Scholar]
- Sullivan L. D., McLoughlin M. G., Goldenberg L. G., Gleave M. E., Marich K. W. Early experience with high-intensity focused ultrasound for the treatment of benign prostatic hypertrophy. Br J Urol. 1997 Feb;79(2):172–176. doi: 10.1046/j.1464-410x.1997.03235.x. [DOI] [PubMed] [Google Scholar]
- Susani M., Madersbacher S., Kratzik C., Vingers L., Marberger M. Morphology of tissue destruction induced by focused ultrasound. Eur Urol. 1993;23 (Suppl 1):34–38. doi: 10.1159/000474677. [DOI] [PubMed] [Google Scholar]
- Zippe C. D. Cryosurgical ablation for prostate cancer: a current review. Semin Urol. 1995 May;13(2):148–156. [PubMed] [Google Scholar]