Abstract
Aim—To evaluate the clinical usefulness of three commercially available assays for Her-2/neu oncogene and protein measurements. The Her-2/neu protein is overexpressed, mostly as a result of gene amplification, in 20–30% of human breast cancers, and has been shown to have prognostic and predictive value for treatment with chemotherapy or the new monoclonal antibody, Herceptin.
Methods—An immunohistochemistry (IHC) assay using the Dako polyclonal antibody A0485, which measures the Her-2/neu protein, was compared with two new Food and Drug Administration (FDA) approved fluorescence in situ hybridisation (FISH) assays—INFORMTM and PathVysionTM, in a cohort of 52 formalin fixed, paraffin wax embedded breast tissues. These tissues were selected randomly from 84 consecutive infiltrating breast cancer specimens, which were first stratified according to the Her-2/neu protein levels as measured by IHC.
Results—The two FISH assays achieved a 98% concordance rate: 14 specimens (27%) showed Her-2/neu gene amplification and 37 specimens (71%) showed no Her-2/neu gene amplification. The PathVysion assay had certain advantages over the INFORM assay. In contrast, the IHC assay detected Her-2/neu overexpression in a high percentage of cases, including 13 high positive specimens (25%) and 13 medium positive specimens (25%). Although 10 of these 13 IHC high positive specimens showed gene amplification by FISH, nine of 13 IHC medium positive specimens showed no gene amplification. Statistical analyses showed that the differences between IHC and FISH assays were primarily in the specimens with medium positive IHC, but negative FISH results.
Conclusions—Because of the increasing importance of the Her-2/neu oncogene and oncoprotein in the clinical management of patients with breast cancer, the accurate and consistent evaluation of Her-2/neu status is crucial. This study suggests that the best approach is to combine both IHC and FISH assays; that is, to use the IHC assay as a triage step, followed by the PathVysion FISH assay to analyse the IHC medium and high positive cases.
Key Words: Her-2/neu • breast cancer • fluorescence in situ hybridisation
Full Text
The Full Text of this article is available as a PDF (212.1 KB).
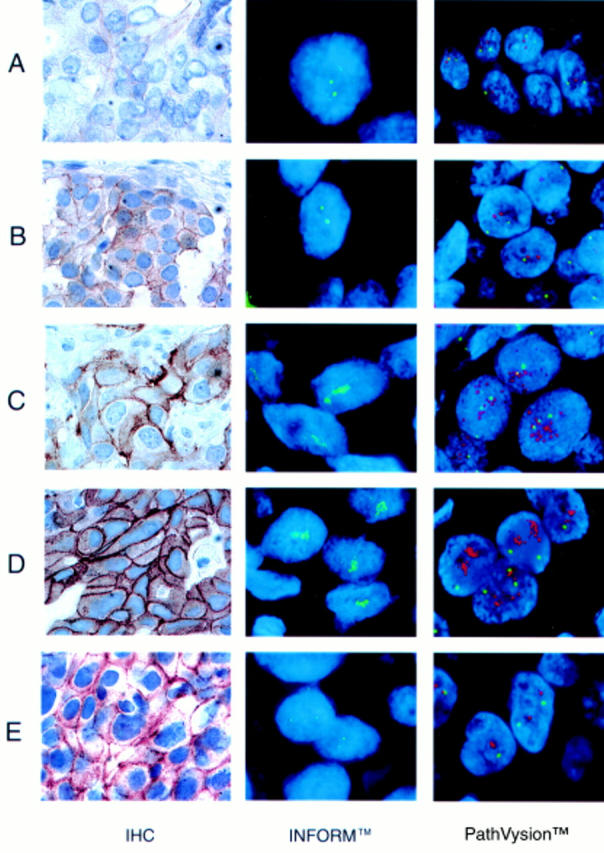
Figure 1 Photomicrograph of the three assays. Representative cases are shown as follows: (A) Immunohistochemistry (IHC) negative and fluorescence in situ hybridisation (FISH; INFORMTM and PathVysionTM) non-amplified; (B) IHC low positive and FISH (INFORM and PathVysion) non-amplified; (C) IHC medium positive and FISH (INFORM and PathVysion) amplified; (D) IHC high positive and FISH (INFORM and PathVysion) amplified; (E) IHC medium positive and FISH (INFORM and PathVysion) non-amplified.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. C., Harvey J. M., Berardo M., Clark G. M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998 Feb;11(2):155–168. [PubMed] [Google Scholar]
- Alroy I., Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997 Jun 23;410(1):83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- Baselga J., Norton L., Albanell J., Kim Y. M., Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998 Jul 1;58(13):2825–2831. [PubMed] [Google Scholar]
- Carter P., Presta L., Gorman C. M., Ridgway J. B., Henner D., Wong W. L., Rowland A. M., Kotts C., Carver M. E., Shepard H. M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson B. A., Gelber R. D., Goldhirsch A., Price K. N., Säve-Söderborgh J., Anbazhagan R., Styles J., Rudenstam C. M., Golouh R., Reed R. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992 Jul;10(7):1049–1056. doi: 10.1200/JCO.1992.10.7.1049. [DOI] [PubMed] [Google Scholar]
- Jacobs T. W., Gown A. M., Yaziji H., Barnes M. J., Schnitt S. J. Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol. 1999 Jul;17(7):1974–1982. doi: 10.1200/JCO.1999.17.7.1974. [DOI] [PubMed] [Google Scholar]
- Jacobs T. W., Gown A. M., Yaziji H., Barnes M. J., Schnitt S. J. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999 Jul;17(7):1983–1987. doi: 10.1200/JCO.1999.17.7.1983. [DOI] [PubMed] [Google Scholar]
- Jimenez R. E., Wallis T., Tabasczka P., Visscher D. W. Determination of Her-2/Neu status in breast carcinoma: comparative analysis of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2000 Jan;13(1):37–45. doi: 10.1038/modpathol.3880007. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Kallioniemi A., Kurisu W., Thor A., Chen L. C., Smith H. S., Waldman F. M., Pinkel D., Gray J. W. ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5321–5325. doi: 10.1073/pnas.89.12.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G. H., Yoon G. S., Lee H. K., Kwon Y. M., Ro J. Y. Clinicopathologic characteristics of replication error-positive gastric carcinoma. Mod Pathol. 1999 Jan;12(1):15–20. [PubMed] [Google Scholar]
- McCann A. H., Dervan P. A., O'Regan M., Codd M. B., Gullick W. J., Tobin B. M., Carney D. N. Prognostic significance of c-erbB-2 and estrogen receptor status in human breast cancer. Cancer Res. 1991 Jun 15;51(12):3296–3303. [PubMed] [Google Scholar]
- Muss H. B., Thor A. D., Berry D. A., Kute T., Liu E. T., Koerner F., Cirrincione C. T., Budman D. R., Wood W. C., Barcos M. c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med. 1994 May 5;330(18):1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- Naber S. P., Tsutsumi Y., Yin S., Zolnay S. A., Mobtaker H., Marks P. J., McKenzie S. J., DeLellis R. A., Wolfe H. J. Strategies for the analysis of oncogene overexpression. Studies of the neu oncogene in breast carcinoma. Am J Clin Pathol. 1990 Aug;94(2):125–136. doi: 10.1093/ajcp/94.2.125. [DOI] [PubMed] [Google Scholar]
- Pauletti G., Godolphin W., Press M. F., Slamon D. J. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene. 1996 Jul 4;13(1):63–72. [PubMed] [Google Scholar]
- Press M. F., Bernstein L., Thomas P. A., Meisner L. F., Zhou J. Y., Ma Y., Hung G., Robinson R. A., Harris C., El-Naggar A. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997 Aug;15(8):2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- Press M. F., Hung G., Godolphin W., Slamon D. J. Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res. 1994 May 15;54(10):2771–2777. [PubMed] [Google Scholar]
- Quénel N., Wafflart J., Bonichon F., de Mascarel I., Trojani M., Durand M., Avril A., Coindre J. M. The prognostic value of c-erbB2 in primary breast carcinomas: a study on 942 cases. Breast Cancer Res Treat. 1995 Sep;35(3):283–291. doi: 10.1007/BF00665980. [DOI] [PubMed] [Google Scholar]
- Reese D. M., Slamon D. J. HER-2/neu signal transduction in human breast and ovarian cancer. Stem Cells. 1997;15(1):1–8. doi: 10.1002/stem.150001. [DOI] [PubMed] [Google Scholar]
- Roche P. C., Ingle J. N. Increased HER2 with U.S. Food and Drug Administration-approved antibody. J Clin Oncol. 1999 Jan;17(1):434–434. doi: 10.1200/JCO.1999.17.1.434. [DOI] [PubMed] [Google Scholar]
- Révillion F., Bonneterre J., Peyrat J. P. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998 May;34(6):791–808. doi: 10.1016/s0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]


