Abstract
Aims/Background—An A to G substitution at base pair 3243 in the mitochondrial tRNA Leu(UUR) gene (mt3243) is commonly associated with maternally inherited diabetes and deafness, and other diseases. It is possible that cell free mitochondrial DNA exists in serum and plasma from these patients, and these samples might be a source of material for the detection of such mutations.
Methods—Sixteen patients with type 2 diabetes mellitus and 25 healthy subjects were tested for the 3243 mutation by polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) analysis. Plasma and serum from the 41 subjects were tested blind, without knowledge of the final diagnosis.
Results—PCR amplification of the mtRNA Leu(UUR) region in mitochondrial DNA (mtDNA) in serum samples revealed the presence of mtDNA in all samples. After ApaI digestion of the amplified DNA fragments, mt3243 was detected in the serum and plasma samples of the seven patients with diabetes who had previously been found to have this mutation in their leucocyte DNA. None of the serum/plasma samples from the healthy subjects or those patients negative for mt3243 in their leucocytes had this mutation (p < 0.001). In addition, the degree of heteroplasmy of mt3243 appeared to be higher in serum and plasma samples than in leucocytes among mt3243 carriers (p < 0.05).
Conclusions—Therefore, mtDNA and associated mutations are present and detectable in serum and plasma. Plasma and serum might be alternative sources for the molecular diagnosis of mt3243 associated diabetes mellitus, as well as other mitochondrial mediated diseases.
Key Words: mitochondrial tRNA Leu(UUR) • mitochondrial 3243 mutation • serum and plasma DNA • type 2 diabetes mellitus
Full Text
The Full Text of this article is available as a PDF (129.2 KB).
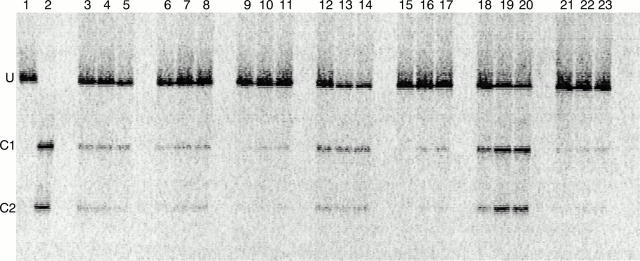
Figure 1 Comparison of the degrees of heteroplasmy detected in blood leucocytes, serum, and plasma in seven patients with diabetes who were positive for mutation mt3243. Lanes 1 and 2, 1% and 100 % mutant mt3243 controls, respectively; lanes 3–5, 6–8, 9–11, 12–14, 15–17, 18–20, and 21–23 are degrees of heteroplasmy of mt3243 in the seven patients with diabetes (M8, M4, M9, M12, M13, M2, and M6, respectively). In each case, results are presented in the order of leucocytes, serum, and plasma samples (from left to right). U denotes the position of the uncleaved wild-type PCR product. C1 and C2 denote the positions of the restriction products of the mutant PCR product.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 't Hart L. M., Jansen J. J., Lemkes H. H., de Knijff P., Maassen J. A. Heteroplasmy levels of a mitochondrial gene mutation associated with diabetes mellitus decrease in leucocyte DNA upon aging. Hum Mutat. 1996;7(3):193–197. doi: 10.1002/(SICI)1098-1004(1996)7:3<193::AID-HUMU2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Amoura Z., Piette J. C., Chabre H., Cacoub P., Papo T., Wechsler B., Bach J. F., Koutouzov S. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis Rheum. 1997 Dec;40(12):2217–2225. doi: 10.1002/art.1780401217. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bell D. A., Morrison B. The spontaneous apoptotic cell death of normal human lymphocytes in vitro: the release of, and immunoproliferative response to, nucleosomes in vitro. Clin Immunol Immunopathol. 1991 Jul;60(1):13–26. doi: 10.1016/0090-1229(91)90108-m. [DOI] [PubMed] [Google Scholar]
- Chen X. Q., Stroun M., Magnenat J. L., Nicod L. P., Kurt A. M., Lyautey J., Lederrey C., Anker P. Microsatellite alterations in plasma DNA of small cell lung cancer patients. Nat Med. 1996 Sep;2(9):1033–1035. doi: 10.1038/nm0996-1033. [DOI] [PubMed] [Google Scholar]
- Ciafaloni E., Ricci E., Servidei S., Shanske S., Silvestri G., Manfredi G., Schon E. A., DiMauro S. Widespread tissue distribution of a tRNALeu(UUR) mutation in the mitochondrial DNA of a patient with MELAS syndrome. Neurology. 1991 Oct;41(10):1663–1664. doi: 10.1212/wnl.41.10.1663. [DOI] [PubMed] [Google Scholar]
- Cooper C. R., Jr, McGinnis M. R. In vitro susceptibility of clinical yeast isolates to fluconazole and terconazole. Am J Obstet Gynecol. 1996 Dec;175(6):1626–1631. doi: 10.1016/s0002-9378(96)70116-3. [DOI] [PubMed] [Google Scholar]
- Goto Y., Horai S., Matsuoka T., Koga Y., Nihei K., Kobayashi M., Nonaka I. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): a correlative study of the clinical features and mitochondrial DNA mutation. Neurology. 1992 Mar;42(3 Pt 1):545–550. doi: 10.1212/wnl.42.3.545. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Johns D. R. The other human genome: mitochondrial DNA and disease. Nat Med. 1996 Oct;2(10):1065–1068. doi: 10.1038/nm1096-1065. [DOI] [PubMed] [Google Scholar]
- Larsson N. G., Tulinius M. H., Holme E., Oldfors A., Andersen O., Wahlström J., Aasly J. Segregation and manifestations of the mtDNA tRNA(Lys) A-->G(8344) mutation of myoclonus epilepsy and ragged-red fibers (MERRF) syndrome. Am J Hum Genet. 1992 Dec;51(6):1201–1212. [PMC free article] [PubMed] [Google Scholar]
- Lo Y. M., Corbetta N., Chamberlain P. F., Rai V., Sargent I. L., Redman C. W., Wainscoat J. S. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997 Aug 16;350(9076):485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- Matthews P. M., Hopkin J., Brown R. M., Stephenson J. B., Hilton-Jones D., Brown G. K. Comparison of the relative levels of the 3243 (A-->G) mtDNA mutation in heteroplasmic adult and fetal tissues. J Med Genet. 1994 Jan;31(1):41–44. doi: 10.1136/jmg.31.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr-Wohlfart U., Rödel G., Henneberg A. Mitochondrial tRNA(Gln) and tRNA(Thr) gene variants in Parkinson's disease. Eur J Med Res. 1997 Mar 24;2(3):111–113. [PubMed] [Google Scholar]
- Mulcahy H. E., Croke D. T., Farthing M. J. Cancer and mutant DNA in blood plasma. Lancet. 1996 Sep 7;348(9028):628–628. doi: 10.1016/S0140-6736(05)65067-2. [DOI] [PubMed] [Google Scholar]
- Nawroz H., Koch W., Anker P., Stroun M., Sidransky D. Microsatellite alterations in serum DNA of head and neck cancer patients. Nat Med. 1996 Sep;2(9):1035–1037. doi: 10.1038/nm0996-1035. [DOI] [PubMed] [Google Scholar]
- Rumore P., Muralidhar B., Lin M., Lai C., Steinman C. R. Haemodialysis as a model for studying endogenous plasma DNA: oligonucleosome-like structure and clearance. Clin Exp Immunol. 1992 Oct;90(1):56–62. doi: 10.1111/j.1365-2249.1992.tb05831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötig A., Bonnefont J. P., Munnich A. Mitochondrial diabetes mellitus. Diabetes Metab. 1996 Oct;22(5):291–298. [PubMed] [Google Scholar]
- Schnopp N. M., Kösel S., Egensperger R., Graeber M. B. Regional heterogeneity of mtDNA heteroplasmy in parkinsonian brain. Clin Neuropathol. 1996 Nov-Dec;15(6):348–352. [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Dronsfield M. J., Mijovic C. H., Hattersley A. T., Yeung V. T., Cockram C., Chan J. C., Barnett A. H., Bain S. C. The mitochondrial tRNA[Leu(UUR)] A to G 3243 mutation is associated with insulin-dependent and non-insulin-dependent diabetes in a Chinese population. Diabet Med. 1997 Dec;14(12):1026–1031. doi: 10.1002/(SICI)1096-9136(199712)14:12<1026::AID-DIA514>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Stroun M., Anker P., Maurice P., Lyautey J., Lederrey C., Beljanski M. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology. 1989;46(5):318–322. doi: 10.1159/000226740. [DOI] [PubMed] [Google Scholar]
- Stroun M., Anker P., Maurice P., Lyautey J., Lederrey C., Beljanski M. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology. 1989;46(5):318–322. doi: 10.1159/000226740. [DOI] [PubMed] [Google Scholar]
- van den Ouweland J. M., Lemkes H. H., Ruitenbeek W., Sandkuijl L. A., de Vijlder M. F., Struyvenberg P. A., van de Kamp J. J., Maassen J. A. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992 Aug;1(5):368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]