Abstract
A number of pigmented lesions are difficult to classify and raise the possibility of a melanoma diagnosis. Care should be exercised to exclude non-melanocytic lesions, and benign melanocytic entities, both of which can mimic melanoma histologically. In addition, the possibility of the lesion being a melanoma variant or epidermotropic metastasis should be considered. There will still be some cases that are difficult to resolve. These usually fall into one of three categories: atypical junctional melanocytic lesion versus early melanoma; naevus versus naevoid melanoma; and atypical Spitz, cellular blue, and deep penetrating naevi versus thick melanoma. These will pose problems even for experts. The atypical Spitz lesions are perhaps the most important category because they tend to be from younger individuals, the differential diagnosis is thick melanoma, and there is no single discriminating histological feature.
Key Words: difficult diagnosis • pigmented lesions • melanoma
Full Text
The Full Text of this article is available as a PDF (266.1 KB).
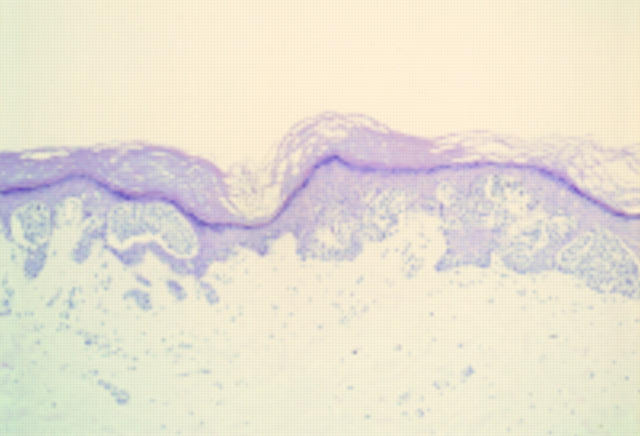
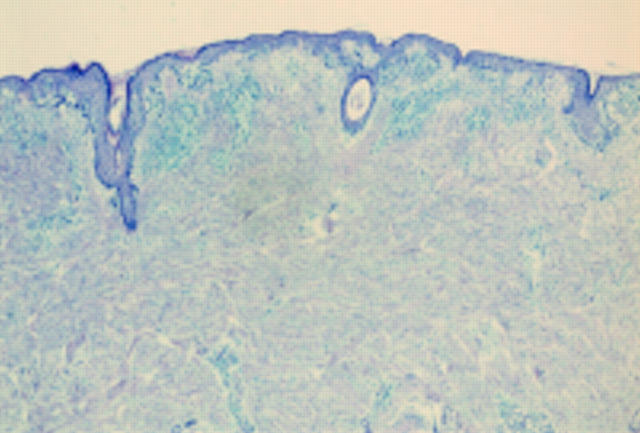
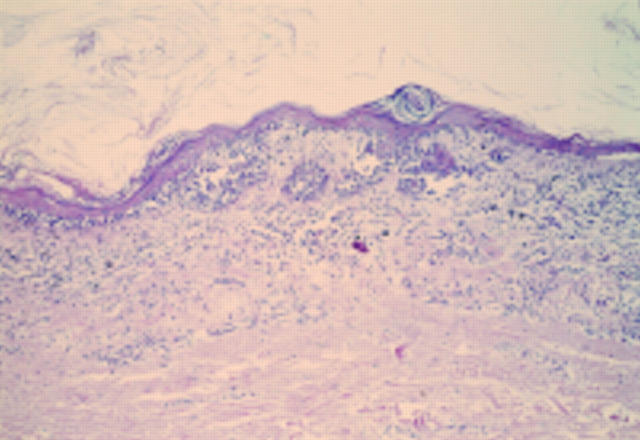
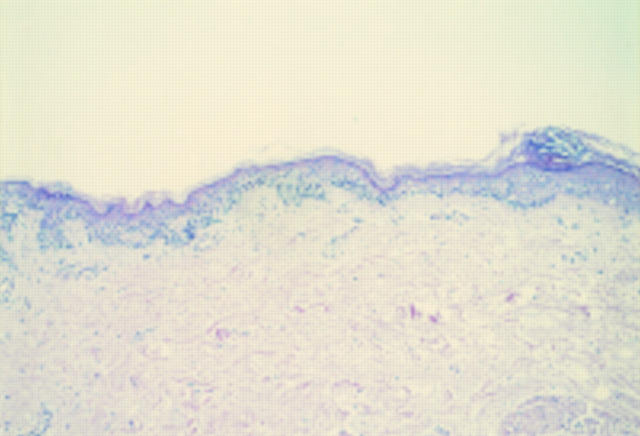
Figure 1 Acral naevus. At low power there is pronounced junctional activity. No psoriasiform hyperplasia is seen.
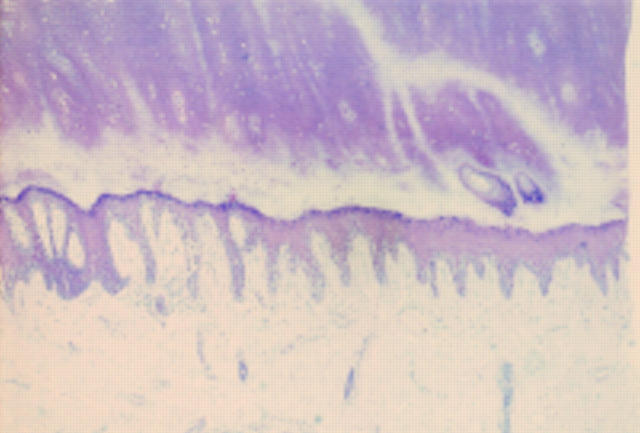
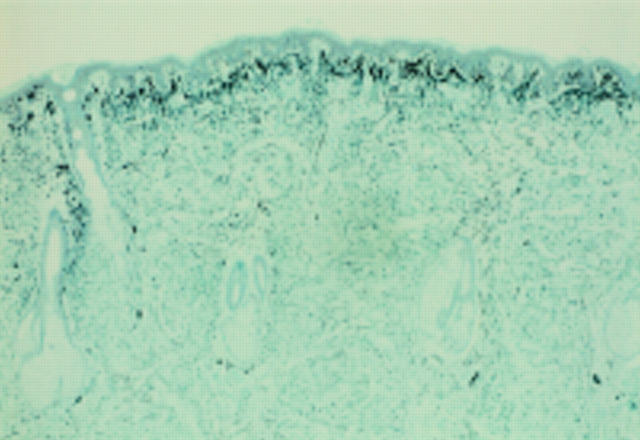
Figure 2 Acral lentiginous melanoma in situ. At low power there is psoriasiform hyperplasia of the epidermis with a subtle lentiginous proliferation of melanocytes along the basal layer.
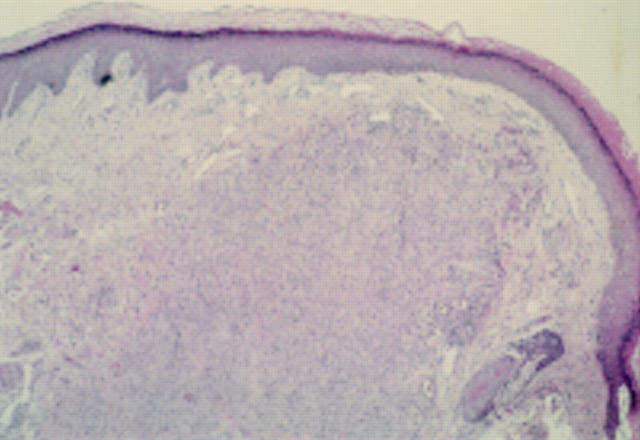
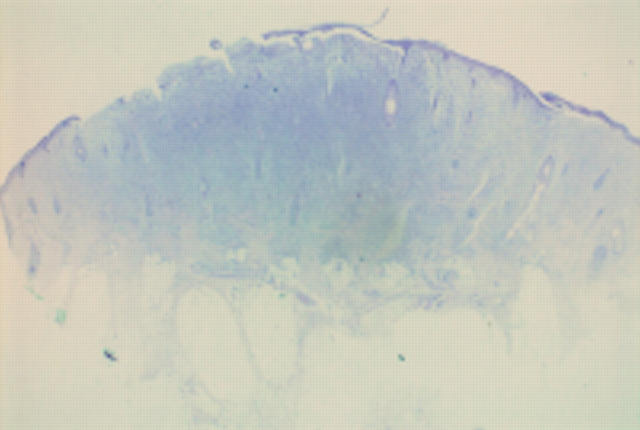
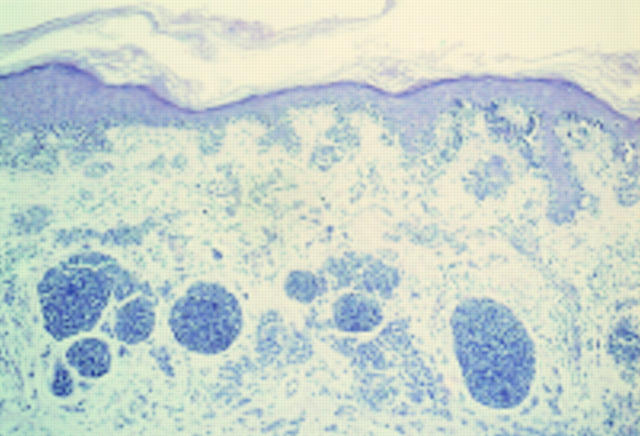
Figure 3 Epithelioid cell histiocytoma. A cellular dermal nodule with a Grenz zone. The lesion extends into deeper tissues.
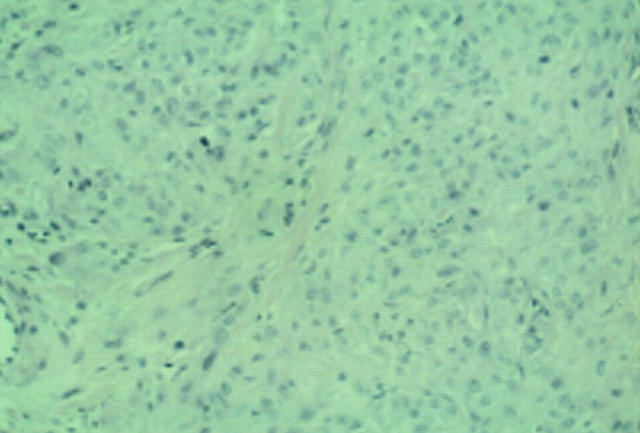
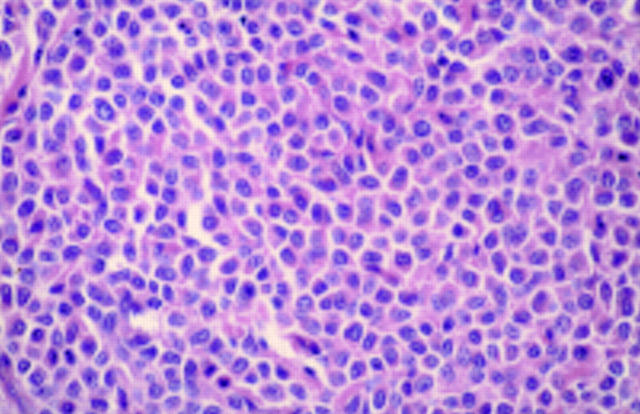
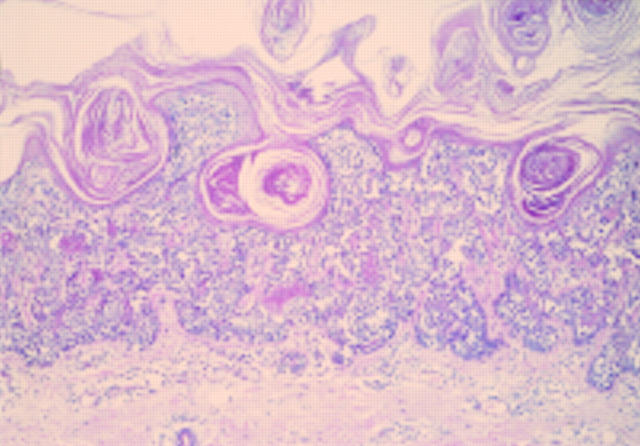
Figure 4 Epithelioid cell histiocytoma. Cells are epithelioid and there is no apparent maturation. A mitotic figure is present. A recent case at the Scottish melanoma group (with permission from Dr KM McLaren, Edinburgh). The differential diagnosis is atypical Spitz, atypical cellular blue naevus, and metastatic melanoma. The lesion was S100 protein negative.
Figure 5 Mastocytoma. A cellular dermal lesion with overlying ulceration and extension of the cells into the fat.
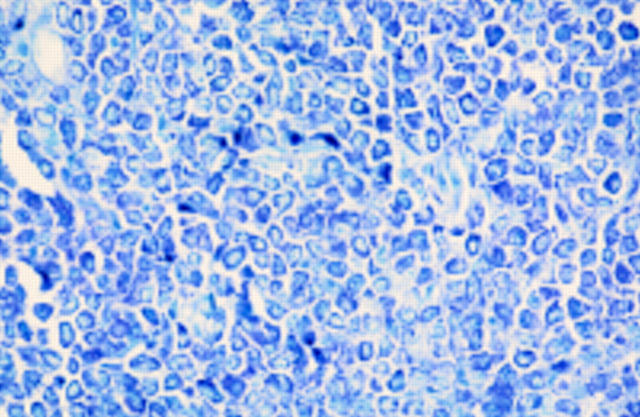
Figure 6 Mastocytoma. Constituent cells of fig 5. The cells have a moderate eosinophilic cytoplasm. There is mild to moderate nuclear atypia.
Figure 7 Mastocytoma. Toluidine blue staining of cells. Cells were S100 protein and Melan-A negative.
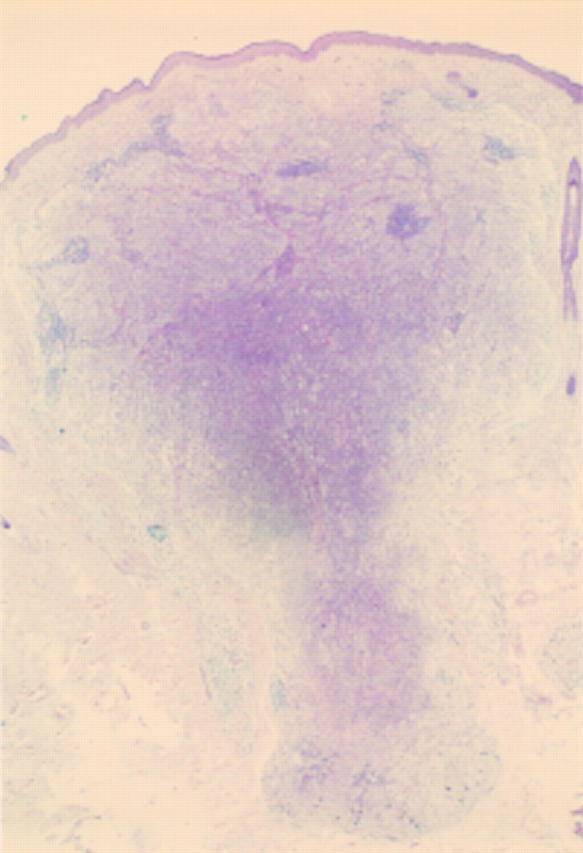
Figure 8 Deep penetrating naevus. Symmetrical dermal lesion extending deeply into the fat. A case from the melanoma slide club (with permission from Dr H Rigby, Bristol). The differential diagnosis is atypical blue naevus and melanoma.
Figure 9 Deep penetrating naevus. The base of the lesion, which is rounded. The cells are growing in a fascicular pattern and there are mild cytological abnormalities. Deep or excessive mitotic activity would raise concerns about the lesion being a melanoma.
Figure 10 Desmoplastic melanoma. A junctional component is obvious at low power, but the dermis appears to be uninvolved.
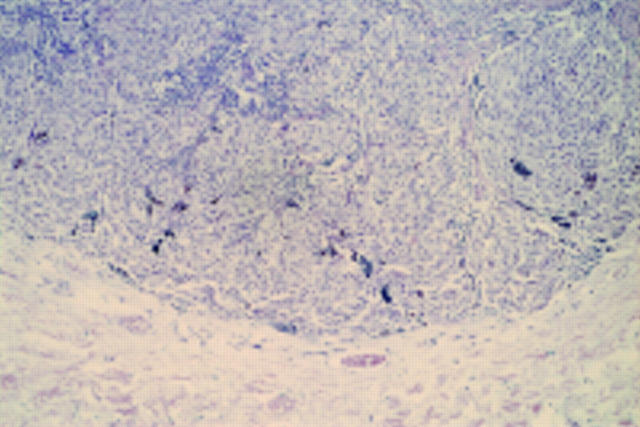
Figure 11 Desmoplastic melanoma. The S100 protein shows the extent of the lesion within the dermis.
Figure 12 Small cell (non-Merkel) melanoma. There is an atypical junctional component and within the dermis there are nests composed of hyperchromatic cells with scanty cytoplasm and mild cytological atypia. There is a proliferation of small blood vessels between the nests and a patchy lymphocytic infiltrate. The patient was in their sixth decade.
Figure 13 Verrucous small cell melanoma. At scanning power there is a warty looking, rather bland melanocytic lesion, composed of small naevoid like cells.
Figure 14 Verrucous small cell melanoma. An area of regression within the lesion.
Figure 15 Verrucous small cell melanoma. The lateral growth spread of the lesion. Taken together with the age of the patient, the regression favoured a malignant diagnosis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avidor I., Kessler E. 'Atypical' blue nevus--a benign variant of cellular blue nevus. Presentation of three cases. Dermatologica. 1977;154(1):39–44. [PubMed] [Google Scholar]
- BEDNAR B. Storiform neurofibromas of the skin, pigmented and nonpigmented. Cancer. 1957 Mar-Apr;10(2):368–376. doi: 10.1002/1097-0142(195703/04)10:2<368::aid-cncr2820100218>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ball N. J., Golitz L. E. Melanocytic nevi with focal atypical epithelioid cell components: a review of seventy-three cases. J Am Acad Dermatol. 1994 May;30(5 Pt 1):724–729. doi: 10.1016/s0190-9622(08)81502-2. [DOI] [PubMed] [Google Scholar]
- Barnhill R. L. Childhood melanoma. Semin Diagn Pathol. 1998 Aug;15(3):189–194. [PubMed] [Google Scholar]
- Barnhill R. L., Dickersin G. R., Nickeleit V., Bhan A. K., Muhlbauer J. E., Phillips M. E., Mihm M. C., Jr Studies on the cellular origin of neurothekeoma: clinical, light microscopic, immunohistochemical, and ultrastructural observations. J Am Acad Dermatol. 1991 Jul;25(1 Pt 1):80–88. doi: 10.1016/0190-9622(91)70177-4. [DOI] [PubMed] [Google Scholar]
- Barnhill R. L., Flotte T. J., Fleischli M., Perez-Atayde A. Cutaneous melanoma and atypical Spitz tumors in childhood. Cancer. 1995 Nov 15;76(10):1833–1845. doi: 10.1002/1097-0142(19951115)76:10<1833::aid-cncr2820761024>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Barnhill R. L., Mihm M. C., Jr, Magro C. M. Plexiform spindle cell naevus: a distinctive variant of plexiform melanocytic naevus. Histopathology. 1991 Mar;18(3):243–247. doi: 10.1111/j.1365-2559.1991.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Barr R. J., Morales R. V., Graham J. H. Desmoplastic nevus: a distinct histologic variant of mixed spindle cell and epithelioid cell nevus. Cancer. 1980 Aug 1;46(3):557–564. doi: 10.1002/1097-0142(19800801)46:3<557::aid-cncr2820460323>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Blessing K. Benign atypical naevi: diagnostic difficulties and continued controversy. Histopathology. 1999 Mar;34(3):189–198. doi: 10.1046/j.1365-2559.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Blessing K., Evans A. T., al-Nafussi A. Verrucous naevoid and keratotic malignant melanoma: a clinico-pathological study of 20 cases. Histopathology. 1993 Nov;23(5):453–458. doi: 10.1111/j.1365-2559.1993.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Blessing K. Malignant melanoma in stasis dermatitis. Histopathology. 1997 Feb;30(2):135–139. doi: 10.1046/j.1365-2559.1997.d01-586.x. [DOI] [PubMed] [Google Scholar]
- Blessing K., McLaren K. M. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992 Apr;20(4):315–322. doi: 10.1111/j.1365-2559.1992.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Blessing K., Sanders D. S., Grant J. J. Comparison of immunohistochemical staining of the novel antibody melan-A with S100 protein and HMB-45 in malignant melanoma and melanoma variants. Histopathology. 1998 Feb;32(2):139–146. doi: 10.1046/j.1365-2559.1998.00312.x. [DOI] [PubMed] [Google Scholar]
- Bruijn J. A., Mihm M. C., Jr, Barnhill R. L. Desmoplastic melanoma. Histopathology. 1992 Mar;20(3):197–205. doi: 10.1111/j.1365-2559.1992.tb00957.x. [DOI] [PubMed] [Google Scholar]
- Busam K. J., Chen Y. T., Old L. J., Stockert E., Iversen K., Coplan K. A., Rosai J., Barnhill R. L., Jungbluth A. A. Expression of melan-A (MART1) in benign melanocytic nevi and primary cutaneous malignant melanoma. Am J Surg Pathol. 1998 Aug;22(8):976–982. doi: 10.1097/00000478-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Busam K. J. Metastatic melanoma to the skin simulating blue nevus. Am J Surg Pathol. 1999 Mar;23(3):276–282. doi: 10.1097/00000478-199903000-00005. [DOI] [PubMed] [Google Scholar]
- Calonje E., Wadden C., Wilson-Jones E., Fletcher C. D. Spindle-cell non-pleomorphic atypical fibroxanthoma: analysis of a series and delineation of a distinctive variant. Histopathology. 1993 Mar;22(3):247–254. doi: 10.1111/j.1365-2559.1993.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Calonje E., Wilson-Jones E., Smith N. P., Fletcher C. D. Cellular 'neurothekeoma': an epithelioid variant of pilar leiomyoma? Morphological and immunohistochemical analysis of a series. Histopathology. 1992 May;20(5):397–404. doi: 10.1111/j.1365-2559.1992.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Carney J. A., Ferreiro J. A. The epithelioid blue nevus. A multicentric familial tumor with important associations, including cardiac myxoma and psammomatous melanotic schwannoma. Am J Surg Pathol. 1996 Mar;20(3):259–272. doi: 10.1097/00000478-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Casso E. M., Grin-Jorgensen C. M., Grant-Kels J. M. Spitz nevi. J Am Acad Dermatol. 1992 Dec;27(6 Pt 1):901–913. doi: 10.1016/0190-9622(92)70286-o. [DOI] [PubMed] [Google Scholar]
- Chan G. S., Choy C., Ng W. K., Chan K. W. Desmoplastic malignant melanoma on the buttock of an 18-year-old girl: differentiation from desmoplastic nevus. Am J Dermatopathol. 1999 Apr;21(2):170–173. doi: 10.1097/00000372-199904000-00011. [DOI] [PubMed] [Google Scholar]
- Clark W. H., Jr, Mastrangelo M. J., Ainsworth A. M., Berd D., Bellet R. E., Bernardino E. A. Current concepts of the biology of human cutaneous malignant melanoma. Adv Cancer Res. 1977;24:267–338. doi: 10.1016/s0065-230x(08)61017-9. [DOI] [PubMed] [Google Scholar]
- Cochran A. J., Bailly C., Paul E., Dolbeau D. Nevi, other than dysplastic and Spitz nevi. Semin Diagn Pathol. 1993 Feb;10(1):3–17. [PubMed] [Google Scholar]
- Collina G., Deen S., Cliff S., Jackson P., Cook M. G. Atypical dermal nodules in benign melanocytic naevi. Histopathology. 1997 Jul;31(1):97–101. doi: 10.1046/j.1365-2559.1997.5960830.x. [DOI] [PubMed] [Google Scholar]
- Conley J., Lattes R., Orr W. Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma). Cancer. 1971 Oct;28(4):914–936. doi: 10.1002/1097-0142(1971)28:4<914::aid-cncr2820280415>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cooper P. H. Deep penetrating (plexiform spindle cell) nevus. A frequent participant in combined nevus. J Cutan Pathol. 1992 Jun;19(3):172–180. doi: 10.1111/j.1600-0560.1992.tb01655.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Cascajo C., Borghi S., Bonczkowitz M. Pigmented atypical fibroxanthoma. Histopathology. 1998 Dec;33(6):537–541. doi: 10.1046/j.1365-2559.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- Echevarria R., Ackerman L. V. Spindle and epitheloid cell nevi in the adult. Clinicopathologic report of 26 cases. Cancer. 1967 Feb;20(2):175–189. doi: 10.1002/1097-0142(1967)20:2<175::aid-cncr2820200203>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Farmer E. R., Gonin R., Hanna M. P. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol. 1996 Jun;27(6):528–531. doi: 10.1016/s0046-8177(96)90157-4. [DOI] [PubMed] [Google Scholar]
- Fletcher C. D., Chan J. K., McKee P. H. Dermal nerve sheath myxoma: a study of three cases. Histopathology. 1986 Feb;10(2):135–145. doi: 10.1111/j.1365-2559.1986.tb02469.x. [DOI] [PubMed] [Google Scholar]
- Gallager R. L., Helwig E. B. Neurothekeoma--a benign cutaneous tumor of neural origin. Am J Clin Pathol. 1980 Dec;74(6):759–764. doi: 10.1093/ajcp/74.6.759. [DOI] [PubMed] [Google Scholar]
- Glusac E. J., Barr R. J., Everett M. A., Pitha J., Santa Cruz D. J. Epithelioid cell histiocytoma. A report of 10 cases including a new cellular variant. Am J Surg Pathol. 1994 Jun;18(6):583–590. [PubMed] [Google Scholar]
- Glusac E. J., McNiff J. M. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999 Feb;21(1):1–7. doi: 10.1097/00000372-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Goldenhersh M. A., Savin R. C., Barnhill R. L., Stenn K. S. Malignant blue nevus. Case report and literature review. J Am Acad Dermatol. 1988 Oct;19(4):712–722. doi: 10.1016/s0190-9622(88)70227-3. [DOI] [PubMed] [Google Scholar]
- Heenan P. J., Clay C. D. Epidermotropic metastatic melanoma simulating multiple primary melanomas. Am J Dermatopathol. 1991 Aug;13(4):396–402. doi: 10.1097/00000372-199108000-00011. [DOI] [PubMed] [Google Scholar]
- House N. S., Fedok F., Maloney M. E., Helm K. F. Malignant melanoma with clinical and histologic features of Merkel cell carcinoma. J Am Acad Dermatol. 1994 Nov;31(5 Pt 2):839–842. doi: 10.1016/s0190-9622(94)70241-1. [DOI] [PubMed] [Google Scholar]
- Jones E. W., Cerio R., Smith N. P. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989 Feb;120(2):185–195. doi: 10.1111/j.1365-2133.1989.tb07782.x. [DOI] [PubMed] [Google Scholar]
- KEMPSON R. L., MCGAVRAN M. H. ATYPICAL FIBROXANTHOMAS OF THE SKIN. Cancer. 1964 Nov;17:1463–1471. doi: 10.1002/1097-0142(196411)17:11<1463::aid-cncr2820171114>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kao G. F., Helwig E. B., Graham J. H. Balloon cell malignant melanoma of the skin. A clinicopathologic study of 34 cases with histochemical, immunohistochemical, and ultrastructural observations. Cancer. 1992 Jun 15;69(12):2942–2952. doi: 10.1002/1097-0142(19920615)69:12<2942::aid-cncr2820691213>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kempf W., Haeffner A. C., Mueller B., Panizzon R. G., Burg G. Experts and gold standards in dermatopathology: qualitative and quantitative analysis of the self-assessment slide seminar at the 17th colloquium of the International Society of Dermatopathology. Am J Dermatopathol. 1998 Oct;20(5):478–482. doi: 10.1097/00000372-199810000-00009. [DOI] [PubMed] [Google Scholar]
- Kerl H., Soyer H. P., Cerroni L., Wolf I. H., Ackerman A. B. Ancient melanocytic nevus. Semin Diagn Pathol. 1998 Aug;15(3):210–215. [PubMed] [Google Scholar]
- Kornberg R., Ackerman A. B. Pseudomelanoma: recurrent melanocytic nevus following partial surgical removal. Arch Dermatol. 1975 Dec;111(12):1588–1590. doi: 10.1001/archderm.111.12.1588. [DOI] [PubMed] [Google Scholar]
- Kossard S., Wilkinson B. Nucleolar organizer regions and image analysis nuclear morphometry of small cell (nevoid) melanoma. J Cutan Pathol. 1995 Apr;22(2):132–136. doi: 10.1111/j.1600-0560.1995.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Kuehnl-Petzoldt C., Berger H., Wiebelt H. Verrucous-keratotic variations of malignant melanoma: a clinicopathological study. Am J Dermatopathol. 1982 Oct;4(5):403–410. [PubMed] [Google Scholar]
- Levene A. On the histological diagnosis and prognosis of malignant melanoma. J Clin Pathol. 1980 Feb;33(2):101–124. doi: 10.1136/jcp.33.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D. M., Wilson-Jones E. Pacinian neurofibroma. Histopathology. 1977 Jul;1(4):247–255. doi: 10.1111/j.1365-2559.1977.tb01664.x. [DOI] [PubMed] [Google Scholar]
- Mackie R. M., Doherty V. R. The desmoplastic melanocytic naevus: a distinct histological entity. Histopathology. 1992 Mar;20(3):207–211. doi: 10.1111/j.1365-2559.1992.tb00958.x. [DOI] [PubMed] [Google Scholar]
- McNutt N. S. "Triggered trap": nevoid malignant melanoma. Semin Diagn Pathol. 1998 Aug;15(3):203–209. [PubMed] [Google Scholar]
- Mehregan D. A., Bergeon M. T., Mehregan D. R. Epidermotropic metastatic malignant melanoma. Cutis. 1995 Apr;55(4):225–227. [PubMed] [Google Scholar]
- Mehregan D. A., Mehregan A. H. Deep penetrating nevus. Arch Dermatol. 1993 Mar;129(3):328–331. [PubMed] [Google Scholar]
- Mehregan D. R., Mehregan D. A., Mehregan A. H. Proliferating cell nuclear antigen staining in deep-penetrating nevi. J Am Acad Dermatol. 1995 Oct;33(4):685–687. doi: 10.1016/0190-9622(95)91312-2. [DOI] [PubMed] [Google Scholar]
- Michal M., Baumruk L., Skálová A. Myxoid change within cellular blue naevi: a diagnostic pitfall. Histopathology. 1992 Jun;20(6):527–530. doi: 10.1111/j.1365-2559.1992.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Nakhleh R. E., Wick M. R., Rocamora A., Swanson P. E., Dehner L. P. Morphologic diversity in malignant melanomas. Am J Clin Pathol. 1990 Jun;93(6):731–740. doi: 10.1093/ajcp/93.6.731. [DOI] [PubMed] [Google Scholar]
- Okun M. R. Histological demarcation of lateral borders: an unsupportable criterion for distinguishing malignant melanoma from Spitz naevus and compound naevus. Histopathology. 1998 Aug;33(2):158–162. doi: 10.1046/j.1365-2559.1998.00443.x. [DOI] [PubMed] [Google Scholar]
- Okun M. R. Melanoma resembling spindle and epithelioid cell nevus. Arch Dermatol. 1979 Dec;115(12):1416–1420. [PubMed] [Google Scholar]
- Paniago-Pereira C., Maize J. C., Ackerman A. B. Nevus of large spindle and/or epithelioid cells (Spitz's nevus). Arch Dermatol. 1978 Dec;114(12):1811–1823. [PubMed] [Google Scholar]
- Peters M. S., Goellner J. R. Spitz naevi and malignant melanomas of childhood and adolescence. Histopathology. 1986 Dec;10(12):1289–1302. doi: 10.1111/j.1365-2559.1986.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Phillips M. E., Margolis R. J., Merot Y., Sober A. J., Reed R. J., Muhlbauer J. E., Mihm M. C., Jr The spectrum of minimal deviation melanoma: a clinicopathologic study of 21 cases. Hum Pathol. 1986 Aug;17(8):796–806. doi: 10.1016/s0046-8177(86)80199-x. [DOI] [PubMed] [Google Scholar]
- Piepkorn M. On the nature of histologic observations: the case of the Spitz nevus. J Am Acad Dermatol. 1995 Feb;32(2 Pt 1):248–254. doi: 10.1016/0190-9622(95)90135-3. [DOI] [PubMed] [Google Scholar]
- Pulitzer D. R., Martin P. C., Cohen A. P., Reed R. J. Histologic classification of the combined nevus. Analysis of the variable expression of melanocytic nevi. Am J Surg Pathol. 1991 Dec;15(12):1111–1122. doi: 10.1097/00000478-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Reed R. J., Ichinose H., Clark W. H., Jr, Mihm M. C., Jr Common and uncommon melanocytic nevi and borderline melanomas. Semin Oncol. 1975 Jun;2(2):119–147. [PubMed] [Google Scholar]
- Reed R. J., Leonard D. D. Neurotropic melanoma. A variant of desmoplastic melanoma. Am J Surg Pathol. 1979 Aug;3(4):301–311. doi: 10.1097/00000478-197908000-00002. [DOI] [PubMed] [Google Scholar]
- Reed R. J., Martin P. Variants of melanoma. Semin Cutan Med Surg. 1997 Jun;16(2):137–158. doi: 10.1016/s1085-5629(97)80008-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez H. A., Ackerman L. V. Cellular blue nevus. Clinicopathologic study of forty-five cases. Cancer. 1968 Mar;21(3):393–405. doi: 10.1002/1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Rosati L. A., Fratamico F. C., Eusebi V. Cellular neurothekeoma. Appl Pathol. 1986;4(3):186–191. [PubMed] [Google Scholar]
- SPITZ S. Melanomas of childhood. Am J Pathol. 1948 May;24(3):591–609. [PMC free article] [PubMed] [Google Scholar]
- Schmoeckel C., Castro C. E., Braun-Falco O. Nevoid malignant melanoma. Arch Dermatol Res. 1985;277(5):362–369. doi: 10.1007/BF00509234. [DOI] [PubMed] [Google Scholar]
- Schrader W. A., Helwig E. B. Balloon cell nevi. Cancer. 1967 Sep;20(9):1502–1514. doi: 10.1002/1097-0142(196709)20:9<1502::aid-cncr2820200918>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Seab J. A., Jr, Graham J. H., Helwig E. B. Deep penetrating nevus. Am J Surg Pathol. 1989 Jan;13(1):39–44. doi: 10.1097/00000478-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Singh Gomez C., Calonje E., Fletcher C. D. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994 Feb;24(2):123–129. doi: 10.1111/j.1365-2559.1994.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Skelton H. G., Smith K. J., Laskin W. B., McCarthy W. F., Gagnier J. M., Graham J. H., Lupton G. P. Desmoplastic malignant melanoma. J Am Acad Dermatol. 1995 May;32(5 Pt 1):717–725. doi: 10.1016/0190-9622(95)91448-x. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Barrett T. L., Skelton H. G., 3rd, Lupton G. P., Graham J. H. Spindle cell and epithelioid cell nevi with atypia and metastasis (malignant Spitz nevus). Am J Surg Pathol. 1989 Nov;13(11):931–939. doi: 10.1097/00000478-198911000-00003. [DOI] [PubMed] [Google Scholar]
- Veenhuizen K. C., De Wit P. E., Mooi W. J., Scheffer E., Verbeek A. L., Ruiter D. J. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997 Jul;182(3):266–272. doi: 10.1002/(SICI)1096-9896(199707)182:3<266::AID-PATH812>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Walsh N., Crotty K., Palmer A., McCarthy S. Spitz nevus versus spitzoid malignant melanoma: an evaluation of the current distinguishing histopathologic criteria. Hum Pathol. 1998 Oct;29(10):1105–1112. doi: 10.1016/s0046-8177(98)90421-x. [DOI] [PubMed] [Google Scholar]
- Webb J. N. The histogenesis of nerve sheath myxoma: report of a case with electron microscopy. J Pathol. 1979 Jan;127(1):35–37. doi: 10.1002/path.1711270106. [DOI] [PubMed] [Google Scholar]
- Wong T. Y., Suster S., Duncan L. M., Mihm M. C., Jr Nevoid melanoma: a clinicopathological study of seven cases of malignant melanoma mimicking spindle and epithelioid cell nevus and verrucous dermal nevus. Hum Pathol. 1995 Feb;26(2):171–179. doi: 10.1016/0046-8177(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Zelger B. G., Steiner H., Kutzner H., Maier H., Zelger B. Cellular 'neurothekeoma': an epithelioid variant of dermatofibroma? Histopathology. 1998 May;32(5):414–422. doi: 10.1046/j.1365-2559.1998.00406.x. [DOI] [PubMed] [Google Scholar]