Abstract
Aims—In anaplastic large cell lymphoma (ALCL), the site of origin has been described as an important prognostic factor. Recently, a fusion protein containing anaplastic lymphoma kinase (ALK) was described in systemic nodal ALCL, and shown to be associated with a good prognosis. The aims of this study were to investigate whether the presence of ALK protein differs between ALCL of different sites of origin; to determine whether ALK expression occurs before dissemination to other sites; and, finally, to investigate whether the site of origin remains a prognostic parameter in ALK negative ALCL.
Methods—ALK expression, as detected by immunohistochemistry using the monoclonal antibodies ALK1 and ALKc, was studied in 85 ALCLs from different sites of origin. In 22 patients, ALK expression was studied in multiple biopsies from different sites (including 13 skin, 16 lymph node, and nine other). Overall survival time was analysed using the Kaplan Meier method.
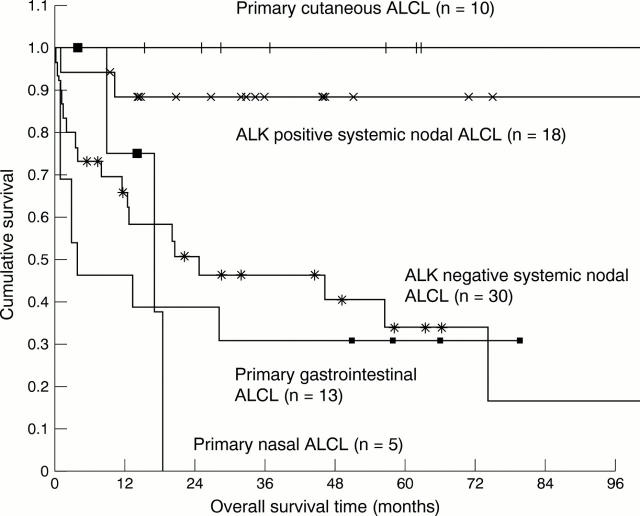
Results—ALK expression was found in 20 of 51 systemic ALCLs with (primary) nodal involvement. No ALK expression was found in 15 primary cutaneous, 14 gastrointestinal, and five nasal ALCLs. Multiple and subsequent biopsies of patients showed ALK expression to be identical to that seen in the primary diagnostic biopsy. Kaplan Meier survival curves showed that in ALK negative ALCLs originating from different sites, primary cutaneous cases are associated with an excellent overall survival, whereas the other cases show a comparable five years survival of less than 40%.
Conclusions—If present, ALK expression favours systemic ALCL with (primary) nodal involvement, and can be used in differentiating between extranodal involvement of systemic (nodal) ALCL and primary extranodal ALCL. ALK is expressed consistently in multiple biopsies of a given patient, indicating that the chromosomal abnormality leading to aberrant ALK expression occurs before dissemination to other sites. Finally, in ALK negative non-cutaneous ALCLs, different sites of origin show comparable poor survival.
Key Words: anaplastic large cell lymphoma • extranodal • anaplastic lymphoma kinase • survival
Full Text
The Full Text of this article is available as a PDF (195.6 KB).
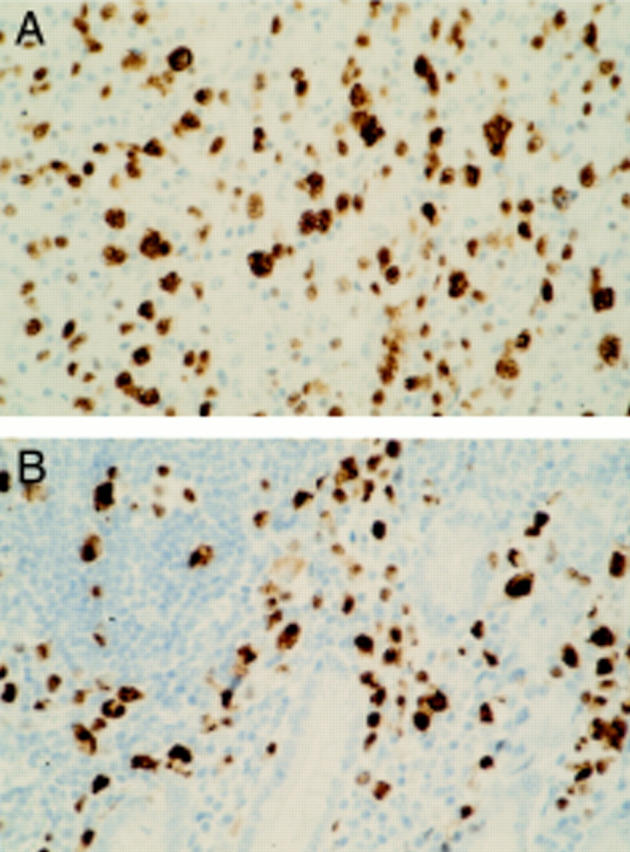
Figure 1 Anaplastic lymphoma kinase (ALK) expression in tumour biopsies of a patient with concurrent nodal (A) and intestinal (B) anaplastic large cell lymphoma. Brown cytoplasmic and nuclear staining of the tumour cells indicates ALK expression, as detected by the monoclonal antibody ALKc. Haematoxylin counterstaining; original magnification, x400.
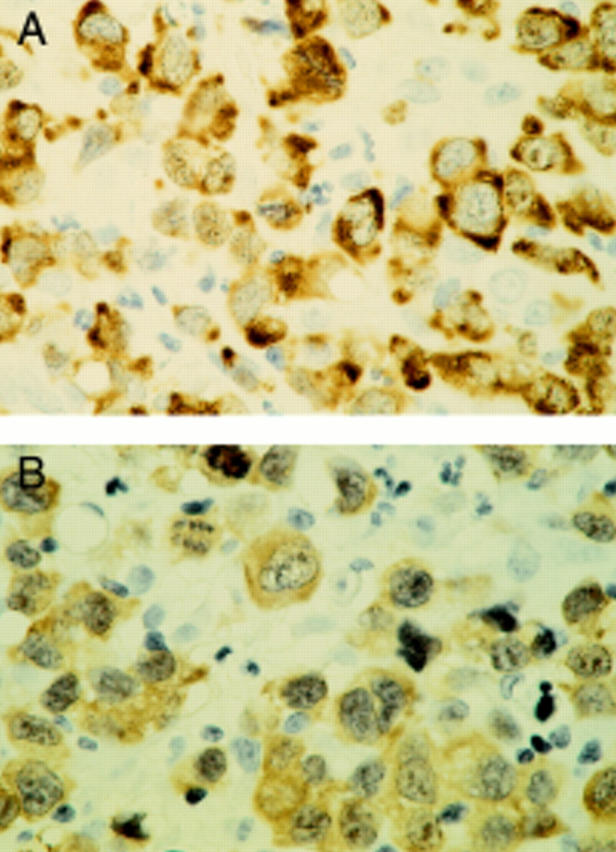
Figure 2 A systemic (nodal) anaplastic large cell lymphoma case with brown, cytoplasm restricted anaplastic lymphoma kinase (ALK) staining of the tumour cells. The cytoplasm restricted staining pattern is the same for monoclonal antibodies ALK1 (A) and ALKc (B). Haematoxylin counterstaining; original magnification, x600.
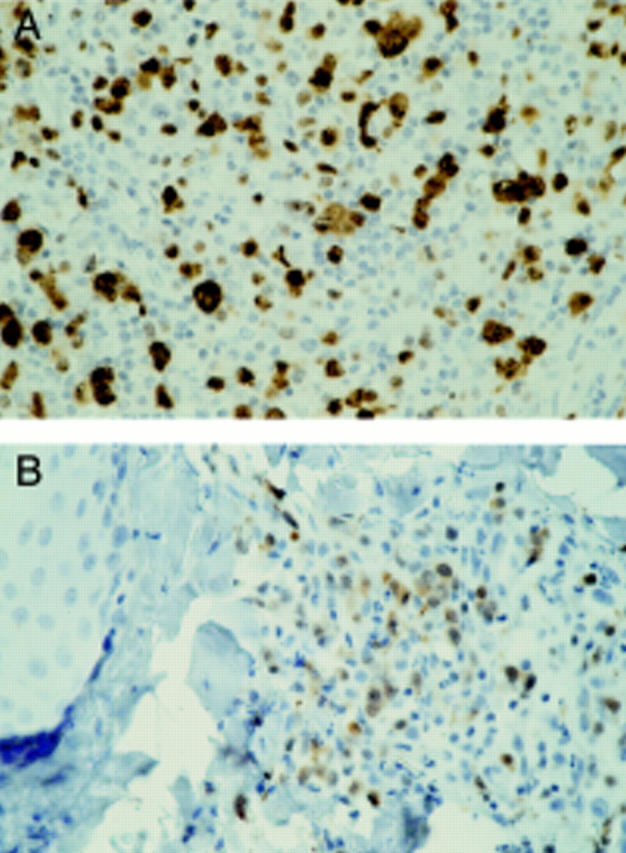
Figure 3 Anaplastic lymphoma kinase (ALK) expression in tumour biopsies of a patient with systemic nodal anaplastic large cell lymphoma (A) and a cutaneous relapse (B). Brown cytoplasmic and nuclear staining of the tumour cells indicates ALK expression, as detected by the monoclonal antibody ALK1. Haematoxylin counterstaining; original magnification, x400.
Figure 4 Comparison of overall survival time in anaplastic large cell lymphoma (ALCL), according to primary site of origin and anaplastic lymphoma kinase (ALK) expression.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benharroch D., Meguerian-Bedoyan Z., Lamant L., Amin C., Brugières L., Terrier-Lacombe M. J., Haralambieva E., Pulford K., Pileri S., Morris S. W. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998 Mar 15;91(6):2076–2084. [PubMed] [Google Scholar]
- Beylot-Barry M., Lamant L., Vergier B., de Muret A., Fraitag S., Delord B., Dubus P., Vaillant L., Delaunay M., MacGrogan G. Detection of t(2;5)(p23;q35) translocation by reverse transcriptase polymerase chain reaction and in situ hybridization in CD30-positive primary cutaneous lymphoma and lymphomatoid papulosis. Am J Pathol. 1996 Aug;149(2):483–492. [PMC free article] [PubMed] [Google Scholar]
- Bischof D., Pulford K., Mason D. Y., Morris S. W. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol. 1997 Apr;17(4):2312–2325. doi: 10.1128/mcb.17.4.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrow M. N., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J Immunol Methods. 1991 Mar 1;137(1):103–112. doi: 10.1016/0022-1759(91)90399-z. [DOI] [PubMed] [Google Scholar]
- DeCoteau J. F., Butmarc J. R., Kinney M. C., Kadin M. E. The t(2;5) chromosomal translocation is not a common feature of primary cutaneous CD30+ lymphoproliferative disorders: comparison with anaplastic large-cell lymphoma of nodal origin. Blood. 1996 Apr 15;87(8):3437–3441. [PubMed] [Google Scholar]
- Falini B., Bigerna B., Fizzotti M., Pulford K., Pileri S. A., Delsol G., Carbone A., Paulli M., Magrini U., Menestrina F. ALK expression defines a distinct group of T/null lymphomas ("ALK lymphomas") with a wide morphological spectrum. Am J Pathol. 1998 Sep;153(3):875–886. doi: 10.1016/S0002-9440(10)65629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B., Pileri S., Zinzani P. L., Carbone A., Zagonel V., Wolf-Peeters C., Verhoef G., Menestrina F., Todeschini G., Paulli M. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999 Apr 15;93(8):2697–2706. [PubMed] [Google Scholar]
- Fujimoto J., Shiota M., Iwahara T., Seki N., Satoh H., Mori S., Yamamoto T. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4181–4186. doi: 10.1073/pnas.93.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoyne R. D., Aoun P., Wu D., Chhanabhai M., Skinnider B. F., Greiner T. C., Morris S. W., Connors J. M., Vose J. M., Viswanatha D. S. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999 Jun 1;93(11):3913–3921. [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Herbst H., Sander C., Tronnier M., Kutzner H., Hügel H., Kaudewitz P. Absence of anaplastic lymphoma kinase (ALK) and Epstein-Barr virus gene products in primary cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis. Br J Dermatol. 1997 Nov;137(5):680–686. [PubMed] [Google Scholar]
- Jaffe E. S., Chan J. K., Su I. J., Frizzera G., Mori S., Feller A. C., Ho F. C. Report of the Workshop on Nasal and Related Extranodal Angiocentric T/Natural Killer Cell Lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996 Jan;20(1):103–111. doi: 10.1097/00000478-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Kinney M. C., Collins R. D., Greer J. P., Whitlock J. A., Sioutos N., Kadin M. E. A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am J Surg Pathol. 1993 Sep;17(9):859–868. doi: 10.1097/00000478-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Kuefer M. U., Look A. T., Pulford K., Behm F. G., Pattengale P. K., Mason D. Y., Morris S. W. Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood. 1997 Oct 15;90(8):2901–2910. [PubMed] [Google Scholar]
- Lamant L., Dastugue N., Pulford K., Delsol G., Mariamé B. A new fusion gene TPM3-ALK in anaplastic large cell lymphoma created by a (1;2)(q25;p23) translocation. Blood. 1999 May 1;93(9):3088–3095. [PubMed] [Google Scholar]
- Lamant L., Meggetto F., al Saati T., Brugières L., de Paillerets B. B., Dastugue N., Bernheim A., Rubie H., Terrier-Lacombe M. J., Robert A. High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin's disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood. 1996 Jan 1;87(1):284–291. [PubMed] [Google Scholar]
- Mason D. Y., Pulford K. A., Bischof D., Kuefer M. U., Butler L. H., Lamant L., Delsol G., Morris S. W. Nucleolar localization of the nucleophosmin-anaplastic lymphoma kinase is not required for malignant transformation. Cancer Res. 1998 Mar 1;58(5):1057–1062. [PubMed] [Google Scholar]
- Morris S. W., Kirstein M. N., Valentine M. B., Dittmer K. G., Shapiro D. N., Saltman D. L., Look A. T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994 Mar 4;263(5151):1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- Ott G., Katzenberger T., Siebert R., DeCoteau J. F., Fletcher J. A., Knoll J. H., Kalla J., Rosenwald A., Ott M. M., Weber-Matthiesen K. Chromosomal abnormalities in nodal and extranodal CD30+ anaplastic large cell lymphomas: infrequent detection of the t(2;5) in extranodal lymphomas. Genes Chromosomes Cancer. 1998 Jun;22(2):114–121. doi: 10.1002/(sici)1098-2264(199806)22:2<114::aid-gcc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Pittaluga S., Wlodarska I., Pulford K., Campo E., Morris S. W., Van den Berghe H., De Wolf-Peeters C. The monoclonal antibody ALK1 identifies a distinct morphological subtype of anaplastic large cell lymphoma associated with 2p23/ALK rearrangements. Am J Pathol. 1997 Aug;151(2):343–351. [PMC free article] [PubMed] [Google Scholar]
- Pulford K., Lamant L., Morris S. W., Butler L. H., Wood K. M., Stroud D., Delsol G., Mason D. Y. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood. 1997 Feb 15;89(4):1394–1404. [PubMed] [Google Scholar]
- Sanchez-Bueno F., Garcia-Marcilla J. A., Alonso J. D., Acosta J., Carrasco L., Piñero A., Parrilla P. Prognostic factors in primary gastrointestinal non-Hodgkin's lymphoma: a multivariate analysis of 76 cases. Eur J Surg. 1998 May;164(5):385–392. doi: 10.1080/110241598750004427. [DOI] [PubMed] [Google Scholar]
- Sandlund J. T., Pui C. H., Roberts W. M., Santana V. M., Morris S. W., Berard C. W., Hutchison R. E., Ribeiro R. C., Mahmoud H., Crist W. M. Clinicopathologic features and treatment outcome of children with large-cell lymphoma and the t(2;5)(p23;q35). Blood. 1994 Oct 15;84(8):2467–2471. [PubMed] [Google Scholar]
- Shiota M., Fujimoto J., Takenaga M., Satoh H., Ichinohasama R., Abe M., Nakano M., Yamamoto T., Mori S. Diagnosis of t(2;5)(p23;q35)-associated Ki-1 lymphoma with immunohistochemistry. Blood. 1994 Dec 1;84(11):3648–3652. [PubMed] [Google Scholar]
- Shiota M., Nakamura S., Ichinohasama R., Abe M., Akagi T., Takeshita M., Mori N., Fujimoto J., Miyauchi J., Mikata A. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood. 1995 Sep 1;86(5):1954–1960. [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Su L. D., Schnitzer B., Ross C. W., Vasef M., Mori S., Shiota M., Mason D. Y., Pulford K., Headington J. T., Singleton T. P. The t(2;5)-associated p80 NPM/ALK fusion protein in nodal and cutaneous CD30+ lymphoproliferative disorders. J Cutan Pathol. 1997 Nov;24(10):597–603. doi: 10.1111/j.1600-0560.1997.tb01090.x. [DOI] [PubMed] [Google Scholar]
- Tilly H., Gaulard P., Lepage E., Dumontet C., Diebold J., Plantier I., Berger F., Symann M., Petrella T., Lederlin P. Primary anaplastic large-cell lymphoma in adults: clinical presentation, immunophenotype, and outcome. Blood. 1997 Nov 1;90(9):3727–3734. [PubMed] [Google Scholar]
- Wellmann A., Doseeva V., Butscher W., Raffeld M., Fukushima P., Stetler-Stevenson M., Gardner K. The activated anaplastic lymphoma kinase increases cellular proliferation and oncogene up-regulation in rat 1a fibroblasts. FASEB J. 1997 Oct;11(12):965–972. doi: 10.1096/fasebj.11.12.9337149. [DOI] [PubMed] [Google Scholar]
- Willemze R., Kerl H., Sterry W., Berti E., Cerroni L., Chimenti S., Diaz-Peréz J. L., Geerts M. L., Goos M., Knobler R. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997 Jul 1;90(1):354–371. [PubMed] [Google Scholar]
- Wlodarska I., De Wolf-Peeters C., Falini B., Verhoef G., Morris S. W., Hagemeijer A., Van den Berghe H. The cryptic inv(2)(p23q35) defines a new molecular genetic subtype of ALK-positive anaplastic large-cell lymphoma. Blood. 1998 Oct 15;92(8):2688–2695. [PubMed] [Google Scholar]
- Zinzani P. L., Magagnoli M., Pagliani G., Bendandi M., Gherlinzoni F., Merla E., Salvucci M., Tura S. Primary intestinal lymphoma: clinical and therapeutic features of 32 patients. Haematologica. 1997 May-Jun;82(3):305–308. [PubMed] [Google Scholar]
- de Bruin P. C., Beljaards R. C., van Heerde P., Van Der Valk P., Noorduyn L. A., Van Krieken J. H., Kluin-Nelemans J. C., Willemze R., Meijer C. J. Differences in clinical behaviour and immunophenotype between primary cutaneous and primary nodal anaplastic large cell lymphoma of T-cell or null cell phenotype. Histopathology. 1993 Aug;23(2):127–135. doi: 10.1111/j.1365-2559.1993.tb00470.x. [DOI] [PubMed] [Google Scholar]
- de Bruin P. C., Noorduyn A. L., van der Valk P., van Heerde P., van Diest P. J., van de Sandt M. M., Ossenkoppele G. J., Meijer C. J. Noncutaneous T-cell lymphomas. Recognition of a lymphoma type (large cell anaplastic) with a relatively favorable prognosis. Cancer. 1993 Apr 15;71(8):2604–2612. doi: 10.1002/1097-0142(19930415)71:8<2604::aid-cncr2820710827>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- ten Berge R. L., Dukers D. F., Oudejans J. J., Pulford K., Ossenkoppele G. J., de Jong D., Miseré J. F., Meijer C. J. Adverse effects of activated cytotoxic T lymphocytes on the clinical outcome of nodal anaplastic large cell lymphoma. Blood. 1999 Apr 15;93(8):2688–2696. [PubMed] [Google Scholar]