Full Text
The Full Text of this article is available as a PDF (139.3 KB).
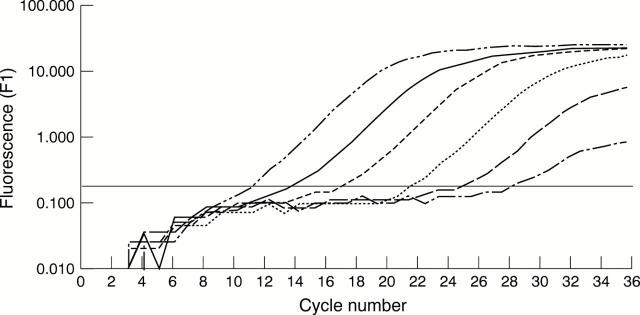
Figure 1 Measurement of hepatitis B virus (HBV) genome in serum using real time monitoring of polymerase chain reaction (PCR) amplification kinetics of a set of standards on the LightCycler instrument. The PCR product is detected by a double stranded DNA specific fluorescent dye. Each line represents a PCR amplification. This experiment demonstrates the amplification of HBV DNA from a 1 log10 dilution series of the HBV DNA positive serum sample standard with an initial concentration of 107 genomes/ml. The concentration of HBV genome is proportionate to the PCR cycle at which the fluorescent signal increases above the background level (represented by the horizontal line).
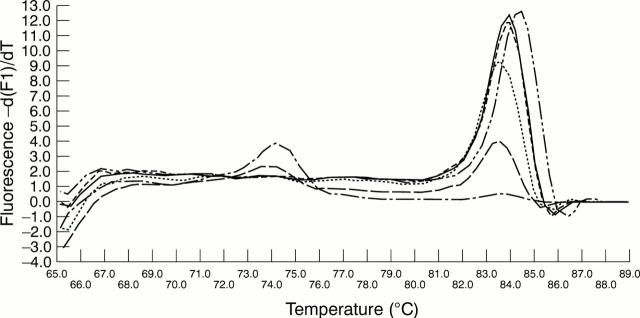
Figure 2 Additional information about the reaction detailed in fig 1 can be obtained from the melting curve analysis. Analysis of the melting characteristics of the polymerase chain reaction (PCR) products demonstrates two distinct products: primer dimer, melting at 74°C, and amplified hepatitis B virus (HBV) product melting at 84°C. Primer dimer formation occurs only at lowest input copy numbers.
Figure 3 A "laboratory on a chip". A DNA chip containing immobilised arrays of oligonucleotides for the study of gene expression. Specialised equipment is required for interpreting the patterns generated by hybridisation of DNA to the array but the technique is suited to automated, high throughput applications.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J. Descartes before the horse: I clone, therefore I am: the hepatitis C virus in current perspective. Ann Intern Med. 1991 Oct 15;115(8):644–649. doi: 10.7326/0003-4819-115-8-644. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Nakatsuji Y., Melpolder J., Wages J., Wesley R., Shih J. W., Kim J. P. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997 Mar 13;336(11):747–754. doi: 10.1056/NEJM199703133361102. [DOI] [PubMed] [Google Scholar]
- Aslanzadeh J., Osmon D. R., Wilhelm M. P., Espy M. J., Smith T. F. A prospective study of the polymerase chain reaction for detection of herpes simplex virus in cerebrospinal fluid submitted to the clinical virology laboratory. Mol Cell Probes. 1992 Oct;6(5):367–373. doi: 10.1016/0890-8508(92)90029-w. [DOI] [PubMed] [Google Scholar]
- Bell H., Hellum K., Harthug S., Maeland A., Ritland S., Myrvang B., von der Lippe B., Raknerud N., Skaug K., Gutigard B. G. Genotype, viral load and age as independent predictors of treatment outcome of interferon-alpha 2a treatment in patients with chronic hepatitis C. Construct group. Scand J Infect Dis. 1997;29(1):17–22. doi: 10.3109/00365549709008658. [DOI] [PubMed] [Google Scholar]
- Burgart L. J., Heller M. J., Reznicek M. J., Greiner T. C., Teneyck C. J., Robinson R. A. Cytomegalovirus detection in bone marrow transplant patients with idiopathic pneumonitis. A clinicopathologic study of the clinical utility of the polymerase chain reaction on open lung biopsy specimen tissue. Am J Clin Pathol. 1991 Nov;96(5):572–576. doi: 10.1093/ajcp/96.5.572. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Cinque P., Brytting M., Vago L., Castagna A., Parravicini C., Zanchetta N., D'Arminio Monforte A., Wahren B., Lazzarin A., Linde A. Epstein-Barr virus DNA in cerebrospinal fluid from patients with AIDS-related primary lymphoma of the central nervous system. Lancet. 1993 Aug 14;342(8868):398–401. doi: 10.1016/0140-6736(93)92814-a. [DOI] [PubMed] [Google Scholar]
- Di Alberti L., Piattelli A., Artese L., Favia G., Patel S., Saunders N., Porter S. R., Scully C. M., Ngui S. L., Teo C. G. Human herpesvirus 8 variants in sarcoid tissues. Lancet. 1997 Dec 6;350(9092):1655–1661. doi: 10.1016/s0140-6736(97)10102-7. [DOI] [PubMed] [Google Scholar]
- Echevarría J. M., Casas I., Tenorio A., de Ory F., Martínez-Martín P. Detection of varicella-zoster virus-specific DNA sequences in cerebrospinal fluid from patients with acute aseptic meningitis and no cutaneous lesions. J Med Virol. 1994 Aug;43(4):331–335. doi: 10.1002/jmv.1890430403. [DOI] [PubMed] [Google Scholar]
- Evans P. C., Soin A., Wreghitt T. G., Alexander G. J. Qualitative and semiquantitative polymerase chain reaction testing for cytomegalovirus DNA in serum allows prediction of CMV related disease in liver transplant recipients. J Clin Pathol. 1998 Dec;51(12):914–921. doi: 10.1136/jcp.51.12.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S. J., Moore P. S. Molecular approaches to the identification of unculturable infectious agents. Emerg Infect Dis. 1996 Jul-Sep;2(3):159–167. doi: 10.3201/eid0203.960301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold D., Rushmore T., Caskey C. T. DNA chips: promising toys have become powerful tools. Trends Biochem Sci. 1999 May;24(5):168–173. doi: 10.1016/s0968-0004(99)01382-1. [DOI] [PubMed] [Google Scholar]
- Guffond T., Dewilde A., Lobert P. E., Caparros-Lefebvre D., Hober D., Wattre P. Significance and clinical relevance of the detection of herpes simplex virus DNA by the polymerase chain reaction in cerebrospinal fluid from patients with presumed encephalitis. Clin Infect Dis. 1994 May;18(5):744–749. doi: 10.1093/clinids/18.5.744. [DOI] [PubMed] [Google Scholar]
- Hawkins A., Davidson F., Simmonds P. Comparison of plasma virus loads among individuals infected with hepatitis C virus (HCV) genotypes 1, 2, and 3 by quantiplex HCV RNA assay versions 1 and 2, Roche Monitor assay, and an in-house limiting dilution method. J Clin Microbiol. 1997 Jan;35(1):187–192. doi: 10.1128/jcm.35.1.187-192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head S. R., Parikh K., Rogers Y. H., Bishai W., Goelet P., Boyce-Jacino M. T. Solid-phase sequence scanning for drug resistance detection in tuberculosis. Mol Cell Probes. 1999 Apr;13(2):81–87. doi: 10.1006/mcpr.1998.0212. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Jeffery K. J., Read S. J., Peto T. E., Mayon-White R. T., Bangham C. R. Diagnosis of viral infections of the central nervous system: clinical interpretation of PCR results. Lancet. 1997 Feb 1;349(9048):313–317. doi: 10.1016/S0140-6736(96)08107-X. [DOI] [PubMed] [Google Scholar]
- Koerner K., Cardoso M., Dengler T., Kerowgan M., Kubanek B. Estimated risk of transmission of hepatitis C virus by blood transfusion. Vox Sang. 1998;74(4):213–216. [PubMed] [Google Scholar]
- Lakeman F. D., Whitley R. J. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995 Apr;171(4):857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- Lipshutz R. J., Fodor S. P., Gingeras T. R., Lockhart D. J. High density synthetic oligonucleotide arrays. Nat Genet. 1999 Jan;21(1 Suppl):20–24. doi: 10.1038/4447. [DOI] [PubMed] [Google Scholar]
- Mellors J. W., Rinaldo C. R., Jr, Gupta P., White R. M., Todd J. A., Kingsley L. A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996 May 24;272(5265):1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Mitchell P. S., Espy M. J., Smith T. F., Toal D. R., Rys P. N., Berbari E. F., Osmon D. R., Persing D. H. Laboratory diagnosis of central nervous system infections with herpes simplex virus by PCR performed with cerebrospinal fluid specimens. J Clin Microbiol. 1997 Nov;35(11):2873–2877. doi: 10.1128/jcm.35.11.2873-2877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. S., Gao S. J., Dominguez G., Cesarman E., Lungu O., Knowles D. M., Garber R., Pellett P. E., McGeoch D. J., Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J Virol. 1996 Jan;70(1):549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutimer D., Pillay D., Dragon E., Tang H., Ahmed M., O'Donnell K., Shaw J., Burroughs N., Rand D., Cane P. High pre-treatment serum hepatitis B virus titre predicts failure of lamivudine prophylaxis and graft re-infection after liver transplantation. J Hepatol. 1999 Apr;30(4):715–721. doi: 10.1016/s0168-8278(99)80204-9. [DOI] [PubMed] [Google Scholar]
- Picciotto A., Campo N., Brizzolara R., Sinelli N., Poggi G., Grasso S., Celle G. HCV-RNA levels play an important role independently of genotype in predicting response to interferon therapy. Eur J Gastroenterol Hepatol. 1997 Jan;9(1):67–69. doi: 10.1097/00042737-199701000-00016. [DOI] [PubMed] [Google Scholar]
- Read S. J., Jeffery K. J., Bangham C. R. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J Clin Microbiol. 1997 Mar;35(3):691–696. doi: 10.1128/jcm.35.3.691-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. J., Kurtz J. B. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J Clin Microbiol. 1999 May;37(5):1352–1355. doi: 10.1128/jcm.37.5.1352-1355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick N., Halaby T., Weverling G. J., Dukers N. H., Simpson G. R., Coutinho R. A., Lange J. M., Schulz T. F., Goudsmit J. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi's sarcoma. AIDS. 1998 Dec 24;12(18):2481–2488. doi: 10.1097/00002030-199818000-00018. [DOI] [PubMed] [Google Scholar]
- Rowley A. H., Whitley R. J., Lakeman F. D., Wolinsky S. M. Rapid detection of herpes-simplex-virus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis. Lancet. 1990 Feb 24;335(8687):440–441. doi: 10.1016/0140-6736(90)90667-t. [DOI] [PubMed] [Google Scholar]
- Schachter J. DFA, EIA, PCR, LCR and other technologies: what tests should be used for diagnosis of chlamydia infections? Immunol Invest. 1997 Jan-Feb;26(1-2):157–161. doi: 10.3109/08820139709048923. [DOI] [PubMed] [Google Scholar]
- Schlesinger Y., Tebas P., Gaudreault-Keener M., Buller R. S., Storch G. A. Herpes simplex virus type 2 meningitis in the absence of genital lesions: improved recognition with use of the polymerase chain reaction. Clin Infect Dis. 1995 Apr;20(4):842–848. doi: 10.1093/clinids/20.4.842. [DOI] [PubMed] [Google Scholar]
- Shiratori Y., Kato N., Yokosuka O., Imazeki F., Hashimoto E., Hayashi N., Nakamura A., Asada M., Kuroda H., Tanaka N. Predictors of the efficacy of interferon therapy in chronic hepatitis C virus infection. Tokyo-Chiba Hepatitis Research Group. Gastroenterology. 1997 Aug;113(2):558–566. doi: 10.1053/gast.1997.v113.pm9247476. [DOI] [PubMed] [Google Scholar]
- Simons J. N., Leary T. P., Dawson G. J., Pilot-Matias T. J., Muerhoff A. S., Schlauder G. G., Desai S. M., Mushahwar I. K. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995 Jun;1(6):564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- Strathdee S. A., Veugelers P. J., Moore P. S. The epidemiology of HIV-associated Kaposi's sarcoma: the unraveling mystery. AIDS. 1996;10 (Suppl A):S51–S57. [PubMed] [Google Scholar]
- Tanel R. E., Kao S. Y., Niemiec T. M., Loeffelholz M. J., Holland D. T., Shoaf L. A., Stucky E. R., Burns J. C. Prospective comparison of culture vs genome detection for diagnosis of enteroviral meningitis in childhood. Arch Pediatr Adolesc Med. 1996 Sep;150(9):919–924. doi: 10.1001/archpedi.1996.02170340033006. [DOI] [PubMed] [Google Scholar]
- Tedder D. G., Ashley R., Tyler K. L., Levin M. J. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann Intern Med. 1994 Sep 1;121(5):334–338. doi: 10.7326/0003-4819-121-5-199409010-00004. [DOI] [PubMed] [Google Scholar]
- The T. H., van der Bij W., van den Berg A. P., van der Giessen M., Weits J., Sprenger H. G., van Son W. J. Cytomegalovirus antigenemia. Rev Infect Dis. 1990 Sep-Oct;12 (Suppl 7):S734–S744. [PubMed] [Google Scholar]
- Thorén A., Widell A. PCR for the diagnosis of enteroviral meningitis. Scand J Infect Dis. 1994;26(3):249–254. doi: 10.3109/00365549409011792. [DOI] [PubMed] [Google Scholar]
- Wolf D. G., Spector S. A. Early diagnosis of human cytomegalovirus disease in transplant recipients by DNA amplification in plasma. Transplantation. 1993 Aug;56(2):330–334. doi: 10.1097/00007890-199308000-00014. [DOI] [PubMed] [Google Scholar]
- Yerly S., Gervaix A., Simonet V., Caflisch M., Perrin L., Wunderli W. Rapid and sensitive detection of enteroviruses in specimens from patients with aseptic meningitis. J Clin Microbiol. 1996 Jan;34(1):199–201. doi: 10.1128/jcm.34.1.199-201.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]