Abstract
Aims—To describe a new fixation and embedding method for tissue samples, immunohistowax processing, which preserves both morphology and antigen immunoreactivity, and to use this technique to investigate the role of dendritic cells in the immune response in peripheral tissues.
Methods—This technique was used to stain a population of specialised antigen presenting cells (dendritic cells) that have the unique capacity to sensitise naive T cells, and therefore to induce primary immune responses. The numbers of dendritic cells in peripheral organs of mice either untreated or injected with live Escherichia coli were compared.
Results—Numbers of dendritic cells were greatly decreased in heart, kidney, and intestine after the inoculation of bacteria. The numbers of dendritic cells in the lung did not seem to be affected by the injection of E coli. However, staining of lung sections revealed that some monocyte like cells acquired morphological and phenotypic features of dendritic cells, and migrated into blood vessels.
Conclusions—These observations suggest that the injection of bacteria induces the activation of dendritic cells in peripheral organs, where they play the role of sentinels, and/or their movement into lymphoid organs, where T cell priming is likely to occur.
Key Words: dendritic cell • Escherichia coli • immunohistochemistry
Full Text
The Full Text of this article is available as a PDF (235.6 KB).
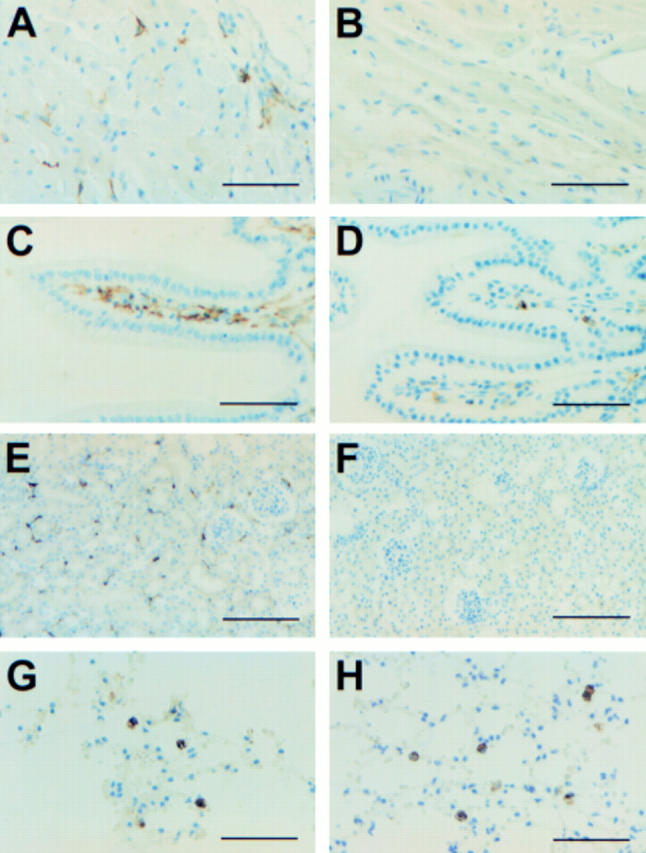
Figure 1 Immunostaining of major histocompatibility complex (MHC) class II positive or CD11c positive cells in organs of mice injected or not injected with bacteria. Heart (A and B), intestine (C and D), kidney (E and F), and lung (G and H). Sections from Balb/c mice injected 24 hours previously with phosphate buffered saline (A, C, E, and G) or Escherichia coli (B, D, F, and H) were stained with anti-I-Ed (A–F) or anti-CD11c (G and H) monoclonal antibodies, and further counterstained with haemalun. Scale bar, 50 µm (A, B, C, D, G, and H), 100 µm (E and F).
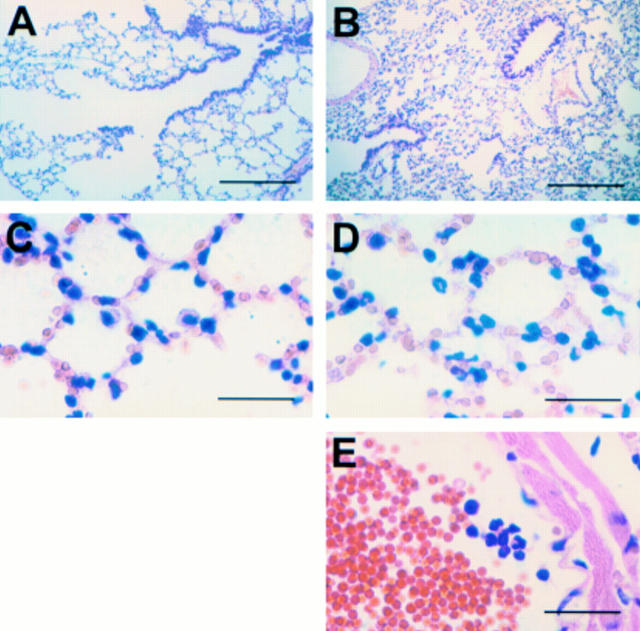
Figure 2 Histopathology of the lung after Escherichia coli injection. Lungs from Balb/c mice injected with phosphate buffered saline (A and C) or E coli (B, D, and E) one hour previously were fixed, dehydrated, and embedded according to the Immunohistowax processing protocol and stained with haemalun-eosin. Note the infiltration of neutrophils in alveoli (D) and in arterioles (E). Scale bars, 250 µm (A and B); 25 µm (C–E).
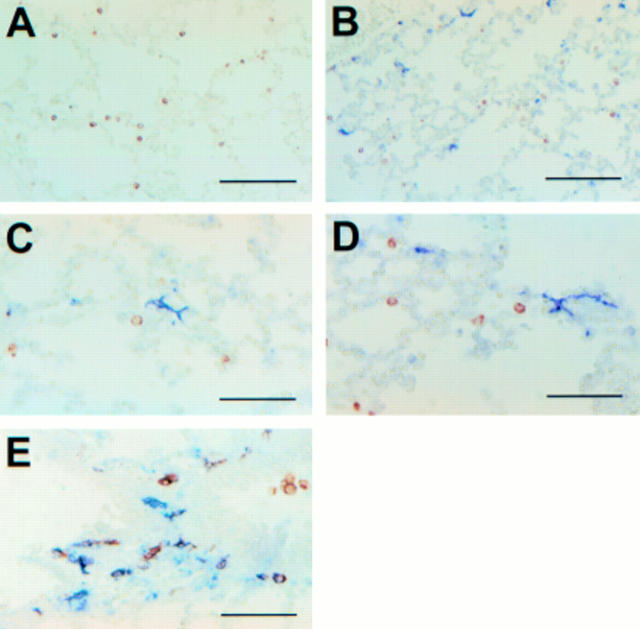
Figure 3 Phenotype and morphology of lung dendritic cells after the inoculation of bacteria. Immunohistowax processed lung sections from mice injected with phosphate buffered saline (A) or live Escherichia coli (B–E) were double stained with anti-CD11c in red and anti-I-Ed in blue. Note the CD11c positive, major histocompatibility complex class II positive cells in purple (B–E). Scale bars, 100 µm (A and B); 50 µm (C–E).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agger R., Witmer-Pack M., Romani N., Stossel H., Swiggard W. J., Metlay J. P., Storozynsky E., Freimuth P., Steinman R. M. Two populations of splenic dendritic cells detected with M342, a new monoclonal to an intracellular antigen of interdigitating dendritic cells and some B lymphocytes. J Leukoc Biol. 1992 Jul;52(1):34–42. doi: 10.1002/jlb.52.1.34. [DOI] [PubMed] [Google Scholar]
- Arnold F. H., Zhang J. H. Metal-mediated protein stabilization. Trends Biotechnol. 1994 May;12(5):189–192. doi: 10.1016/0167-7799(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R. M. Dendritic cells and the control of immunity. Nature. 1998 Mar 19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Christianson D. W. Structural biology of zinc. Adv Protein Chem. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- Coulie P. G., Uyttenhove C., Wauters P., Manolios N., Klausner R. D., Samelson L. E., Van Snick J. Identification of a murine monoclonal antibody specific for an allotypic determinant on mouse CD3. Eur J Immunol. 1991 Jul;21(7):1703–1709. doi: 10.1002/eji.1830210718. [DOI] [PubMed] [Google Scholar]
- De Smedt T., Pajak B., Klaus G. G., Noelle R. J., Urbain J., Leo O., Moser M. Antigen-specific T lymphocytes regulate lipopolysaccharide-induced apoptosis of dendritic cells in vivo. J Immunol. 1998 Nov 1;161(9):4476–4479. [PubMed] [Google Scholar]
- De Smedt T., Pajak B., Muraille E., Lespagnard L., Heinen E., De Baetselier P., Urbain J., Leo O., Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996 Oct 1;184(4):1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbold J., Johnson-Léger C., Atkins C. J., Clark E. A., Klaus G. G. Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur J Immunol. 1994 Aug;24(8):1835–1842. doi: 10.1002/eji.1830240817. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Bradshaw J., Linsley P., Hodes R. J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993 Nov 5;262(5135):905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Pack M. W., Aya H., Inaba M., Sudo T., Wolpe S., Schuler G. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992 May 1;175(5):1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingulli E., Mondino A., Khoruts A., Jenkins M. K. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997 Jun 16;185(12):2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki R., Taniyama Y., Seko C., Nakamura H., Kikuchi M., Ikehara M. Design and creation of a Ca2+ binding site in human lysozyme to enhance structural stability. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6903–6907. doi: 10.1073/pnas.86.18.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J. P., Witmer-Pack M. D., Agger R., Crowley M. T., Lawless D., Steinman R. M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990 May 1;171(5):1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narhi L. O., Philo J. S., Li T., Zhang M., Samal B., Arakawa T. Induction of alpha-helix in the beta-sheet protein tumor necrosis factor-alpha: thermal- and trifluoroethanol-induced denaturation at neutral pH. Biochemistry. 1996 Sep 3;35(35):11447–11453. doi: 10.1021/bi952766v. [DOI] [PubMed] [Google Scholar]
- Paineau J., Priestley C., Fabre J., Chevalier S., van der Meide P., Schellekens H., Jacques Y., Soulillou J. P. Effect of recombinant interferon gamma and interleukin-2 and of a monoclonal antibody against interferon gamma on the rat immune response against heart allografts. J Heart Lung Transplant. 1991 May-Jun;10(3):424–430. [PubMed] [Google Scholar]
- Payne J., Huber B. T., Cannon N. A., Schneider R., Schilham M. W., Acha-Orbea H., MacDonald H. R., Hengartner H. Two monoclonal rat antibodies with specificity for the beta-chain variable region V beta 6 of the murine T-cell receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7695–7698. doi: 10.1073/pnas.85.20.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman G. M., Spencer C., Sperling A. I., Bluestone J. A. Role of gamma delta T cells in murine collagen-induced arthritis. J Immunol. 1993 Dec 1;151(11):6546–6558. [PubMed] [Google Scholar]
- Pudney J., Anderson D. Effects of fixation and paraffin embedding on the immunohistological detection of cell-associated HIV-1 by different monoclonal antibodies. J Histochem Cytochem. 1995 Sep;43(9):857–862. doi: 10.1177/43.9.7543912. [DOI] [PubMed] [Google Scholar]
- Randolph G. J., Beaulieu S., Lebecque S., Steinman R. M., Muller W. A. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998 Oct 16;282(5388):480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- Regan L., Clarke N. D. A tetrahedral zinc(II)-binding site introduced into a designed protein. Biochemistry. 1990 Dec 11;29(49):10878–10883. doi: 10.1021/bi00501a003. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C., Hieny S., Scharton-Kersten T., Jankovic D., Charest H., Germain R. N., Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997 Dec 1;186(11):1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roake J. A., Rao A. S., Morris P. J., Larsen C. P., Hankins D. F., Austyn J. M. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995 Jun 1;181(6):2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Pack M., Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997 Apr;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Roberts V. A., Getzoff E. D. Metal-binding sites in proteins. Curr Opin Biotechnol. 1991 Aug;2(4):582–591. doi: 10.1016/0958-1669(91)90084-i. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Roberts V. A., Getzoff E. D. Protein metal-binding sites. Curr Opin Biotechnol. 1992 Aug;3(4):378–387. doi: 10.1016/0958-1669(92)90166-g. [DOI] [PubMed] [Google Scholar]
- Thomas P. D., Dill K. A. Local and nonlocal interactions in globular proteins and mechanisms of alcohol denaturation. Protein Sci. 1993 Dec;2(12):2050–2065. doi: 10.1002/pro.5560021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Ehl S., Aichele P., Oehen S., Kündig T., Hengartner H. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997 Apr;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- van Stokkum I. H., Linsdell H., Hadden J. M., Haris P. I., Chapman D., Bloemendal M. Temperature-induced changes in protein structures studied by Fourier transform infrared spectroscopy and global analysis. Biochemistry. 1995 Aug 22;34(33):10508–10518. doi: 10.1021/bi00033a024. [DOI] [PubMed] [Google Scholar]